lewis structure for c2h4o

C2h4 Lewis Dot Structure
Formula: C 2 H 4 O Molecular weight: 44.0526 IUPAC Standard InChI: InChI=1S/C2H4O/c1-2-3-1/h1-2H2 IUPAC Standard InChIKey: IAYPIBMASNFSPL-UHFFFAOYSA-N CAS Registry Number: 75-21-8 Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript .
Quimica Organica Etanal
Drawing the Lewis structure for C 2 H 4 (named ethene) requires the use of a double bond. In a double bond two pairs of valence electrons are shared (for a total of four valence electrons). Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. Video.
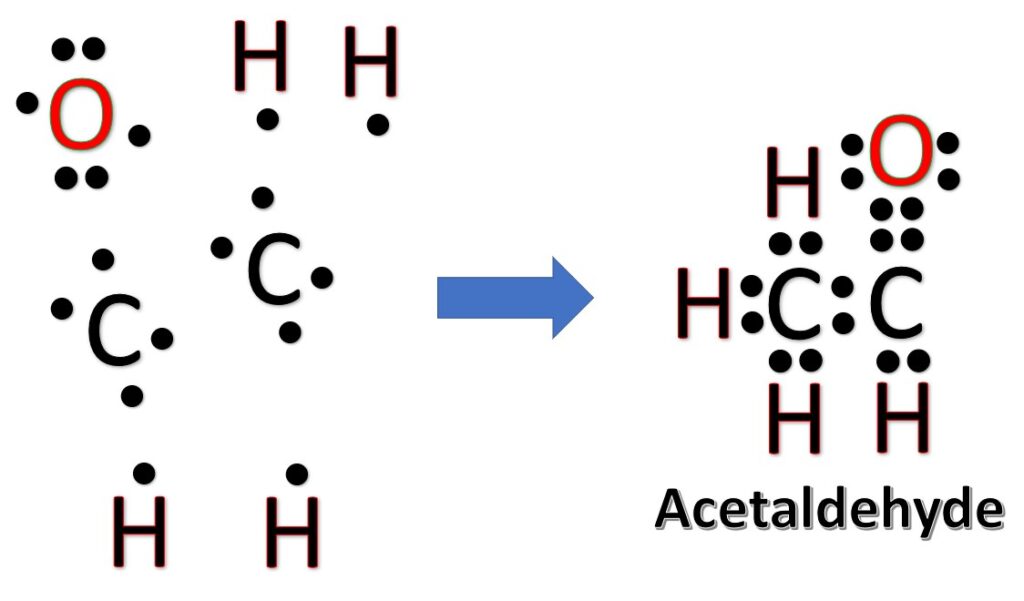
The Lewis Structures of C2H4O [with free study guide and video]
The information on this page is fact-checked. C 2 H 4 Lewis structure. C 2 H 4 (ethylene or ethene) has two carbon atoms and four hydrogen atoms. In the C 2 H 4 Lewis structure, there is a double bond between the two carbon atoms, and each carbon is attached with one hydrogen atom, and none of the atoms has a lone pair.

What is the Lewis dot structure of \ce{C2H4O}? Quizlet
Acetaldehyde (ethanal) Ethenol (vinyl alcohol) Ethylene oxide (epoxyethane, oxirane) This set index page lists chemical structure articles associated with the same molecular formula. If an internal link led you here, you may wish to change the link to point directly to the intended article.

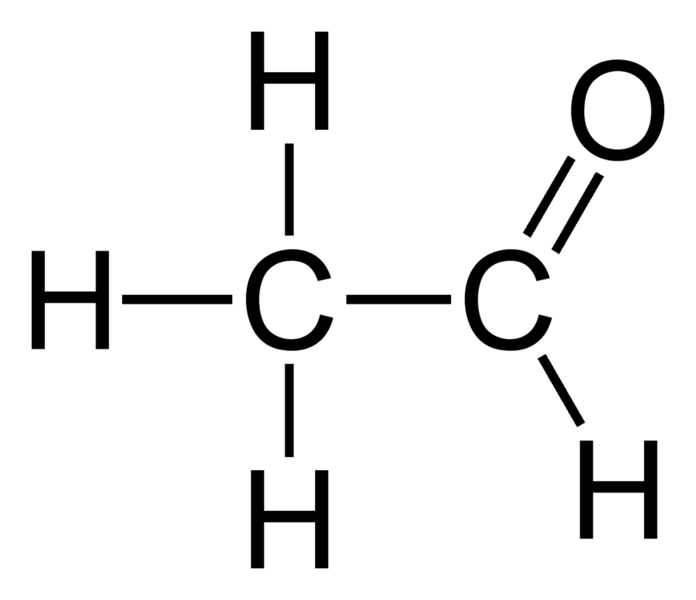
Acetaldehyde Formula Uses, Structures, Examples Embibe
The Lewis structure of acetaldehyde (C2H4O) is made up of 2 carbon (C) atoms present at the center while 4 hydrogens (H) and 1 oxygen (O) atom occupy terminal positions. According to the central C-atom, there are a total of 3 electron density regions around the central atom and it has no lone pair of electrons.
lewis structure for c2h4o
Here's how you can easily draw the C 2 H 2 O Lewis structure step by step: #1 Draw a rough skeleton structure #2 Mention lone pairs on the atoms #3 If needed, mention formal charges on the atoms #4 Minimize formal charges by converting lone pairs of the atoms, and try to get a stable Lewis structure

Acetaldehyde Formula C2H4O Explained, Uses, Structures, Examples
Give the Lewis structure for C 2 H 4 O. This formula has at least two possible structures (isomers). Include those structures. Isomers: Compounds having an identical chemical formula but.

C2H4O Lewis Structure
Ethylene Oxide | C2H4O | CID 6354 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more.

CH4O Lewis Structure How to Draw the Lewis Structure for CH4O YouTube
Draw the Lewis structure for C2H4O2 OneClass 14K subscribers Subscribe Subscribed 4.1K views 2 years ago 🚀To book a personalized 1-on-1 tutoring session: 👉Janine The Tutor.

draw the lewis structure for vinyl alcohol heyjudestrummingpattern
Science Chemistry Chemistry questions and answers Draw the Lewis structure for acetaldehyde (C2H4O). Acetaldehyde molecules have one carbonyl group. Be certain you include any lone pairs. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer
lewis structure for c2h4o
-111 °C OU Chemical Safety Data (No longer updated) More details-111 °C Jean-Claude Bradley Open Melting Point Dataset 16045-111.7 °C (Literature) Jean-Claude Bradley Open Melting Point Dataset 21302-111.7 °C FooDB FDB003358-172--170 °F (-113.3333--112.2222 °C) Wikidata Q407473-171 °F (-112.7778 °C) Wikidata Q407473-111 °C Sigma-Aldrich SIAL-03906

Locating transition states C2H4 + HCl
C2H4O2 Lewis structure: How to Draw the Lewis Structure for C2H4O2 Geometry of Molecules 3.12K subscribers Subscribe Subscribed 1 2 3 4 5 6 7 8 9 0 1 2 3 4 5 6 7 8 9 0 1 2 3 4 5 6 7 8 9 1 2.
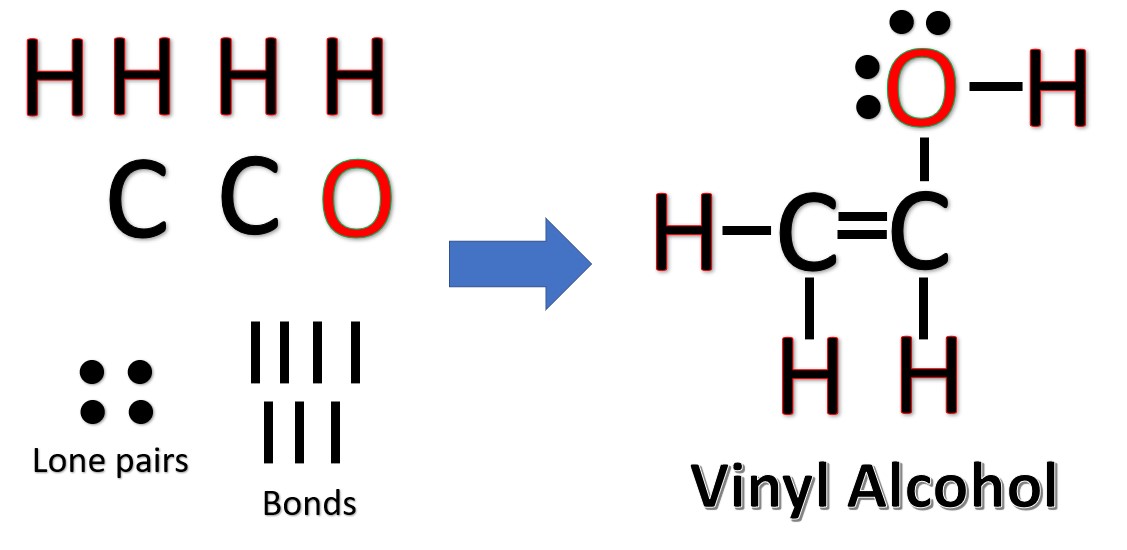
The Lewis Structures of C2H4O [with free study guide and video]
Not to be confused with Ethyl oxide. Ethylene oxide is an organic compound with the formula C2H4O. It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor.

C2H4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram
C2H4 Lewis Structure Hydrogen is the least electronegative element here. However, in Hydrocarbons, we always place the Carbon atoms in the center as shown in the figure. We have 12 available valence electrons. Start by forming covalent bonds between the Carbon and Hydrogen atoms.
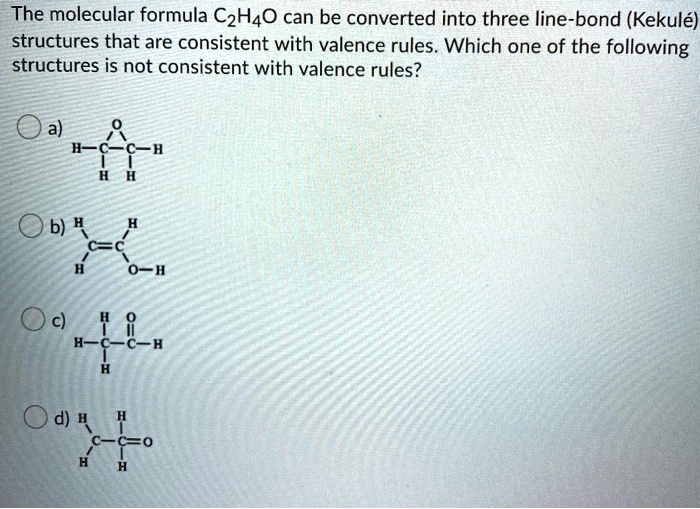
SOLVED The molecular formula C2H4O can be converted into three line
C2H4 Lewis Structure The electron dot structure, widely known as Lewis Structure, is a skeletal diagrammatic representation of a molecule taking into account the constituent atoms and the valence shell electrons.

lewis structure for c2h4o
Geometry of Molecules Hi Guys! C2H4O is a chemical formula for ethylene oxide. It is also known as vinyl alcohol, and to find out its Lewis Structure, we first look at the total n.