Atomic Structure Biochemistry
4.2 Structure of Atoms SPM Science
The electron configuration and orbital diagram of helium are: The n = 1 shell is completely filled in a helium atom. The next atom is the alkali metal lithium with an atomic number of 3. The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium.

Structure of an atom universalxoler
An atom that gains one or more electrons will exhibit a negative charge and is called an anion. Positively charged atoms called cations are formed when an atom loses one or more electrons. For example, a neutral sodium atom (Z = 11) has 11 electrons. If this atom loses one electron, it will become a cation with a 1+ charge (11 − 10 = 1+).
What is Atom? How does it Exist? and it's Symbols Teachoo
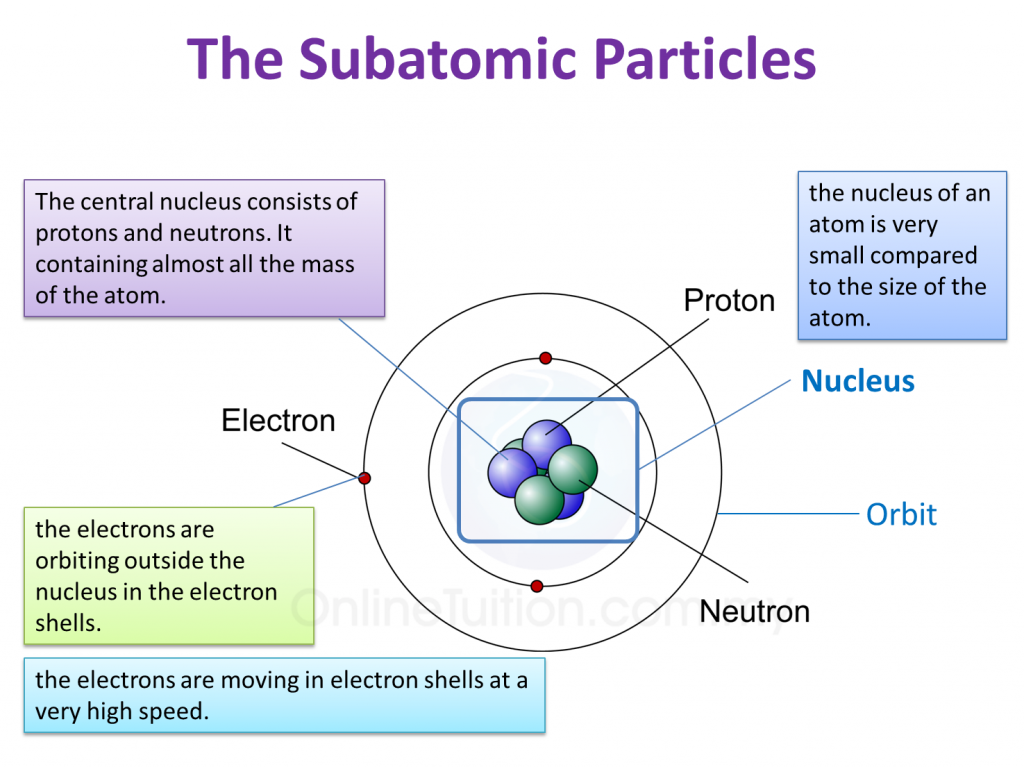
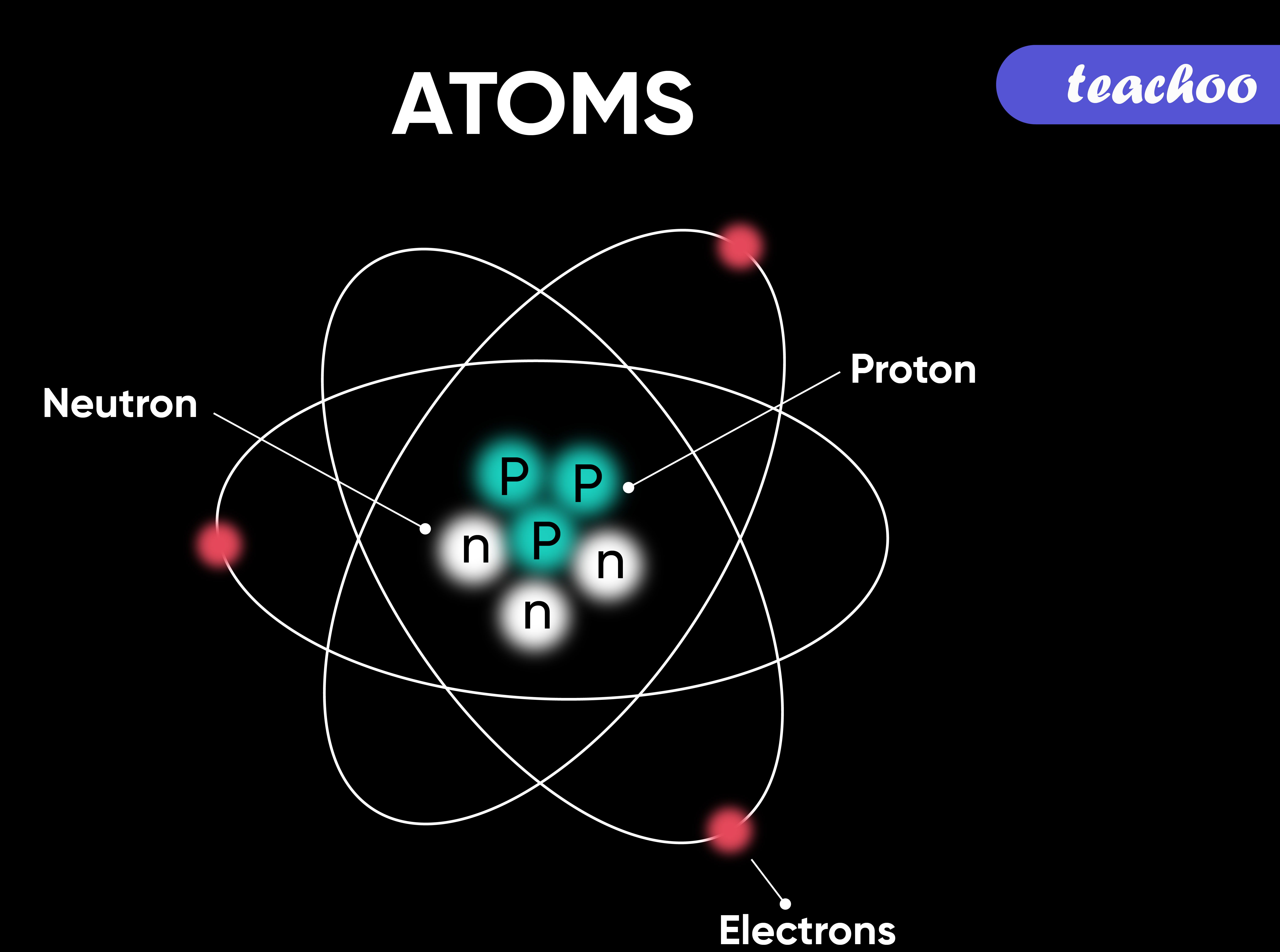
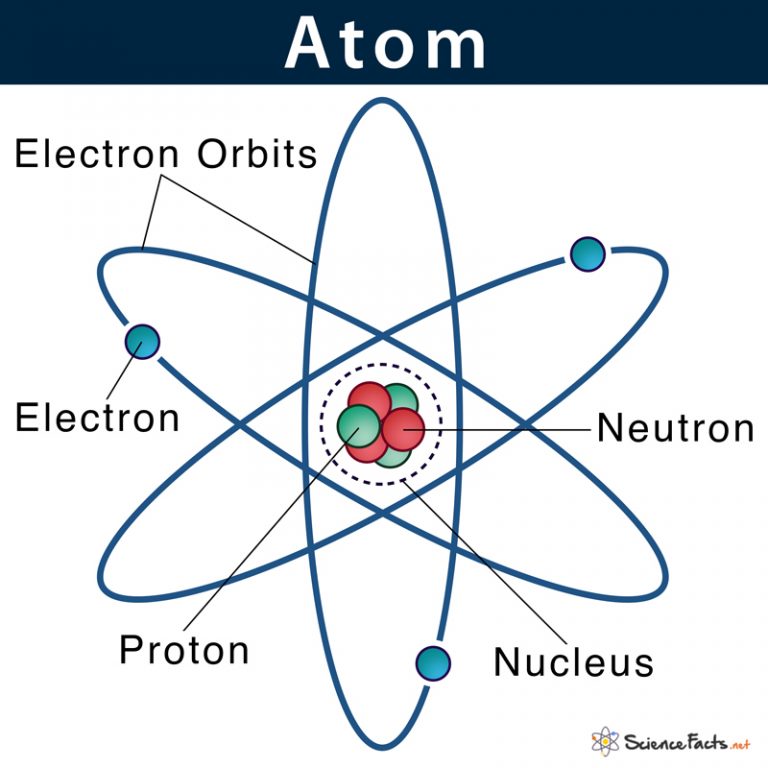
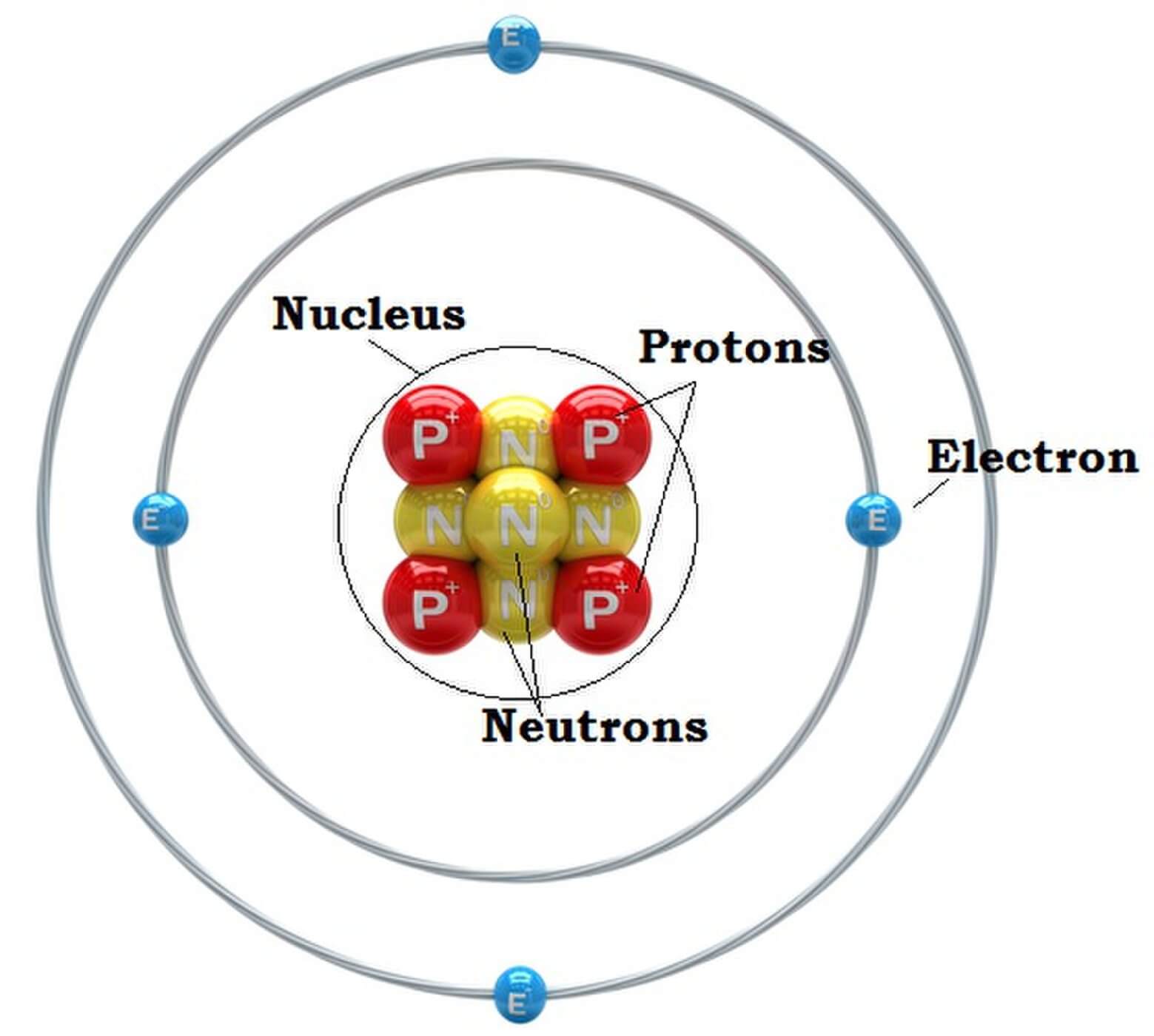
Parts of an Atom An atom consists of two parts. These are the nucleus and extranuclear portions. The nucleus is present in the centre of the atom and is surrounded by the extranuclear portions. The radius of the nucleus of an atom is nearly 10 - 15 m, while that of the atom is 10 - 10 m.
Atom Definition, Structure & Parts with Labeled Diagram
Relative charge. -1. The number of electrons in an atom is always the same as the number of protons, so atoms are electrically. neutral. overall. Atoms can lose or gain electrons. When they do.
What is an Atom? Definitions & Examples Let us learn Basics News Bugz
The periodic table By convention, elements are organized in the periodic table, a structure that captures important patterns in their behavior. Devised by Russian chemist Dmitri Mendeleev (1834-1907) in 1869, the table places elements into columns— groups —and rows— periods —that share certain properties.

Atomic Structure Broad Learnings
Chemical Symbols. A chemical symbol is an abbreviation that we use to indicate an element or an atom of an element. For example, the symbol for mercury is Hg (Figure \(\PageIndex{3}\)). We use the same symbol to indicate one atom of mercury (microscopic domain) or to label a container of many atoms of the element mercury (macroscopic domain).
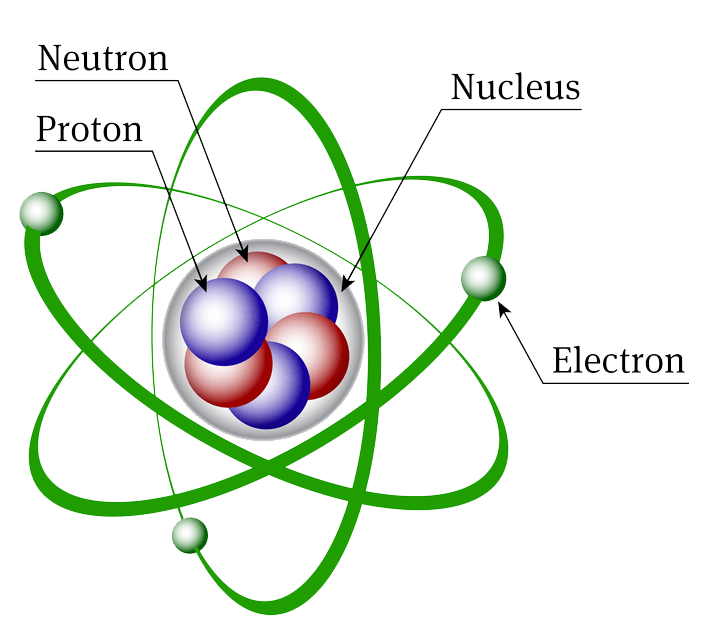
Atomic Structure Biochemistry
GCSE AQA Trilogy Atomic structure - AQA Structure of the atom Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The numbers of subatomic particles.
/GettyImages-141483984-56a133b65f9b58b7d0bcfdb1.jpg)
35 Label The Parts Of The Atom In The Diagram Below Labels For Your Ideas
Atomic or ionic charge = number of protons − number of electrons. An atom that gains one or more electrons will exhibit a negative charge and is called an anion. Positively charged atoms called cations are formed when an atom loses one or more electrons. For example, a neutral sodium atom (Z = 11) has 11 electrons.

Atoms and Atomic Structure
Summary. An atom consists of a small, positively charged nucleus surrounded by electrons. The nucleus contains protons and neutrons; its diameter is about 100,000 times smaller than that of the atom. The mass of one atom is usually expressed in atomic mass units (amu), which is referred to as the atomic mass.
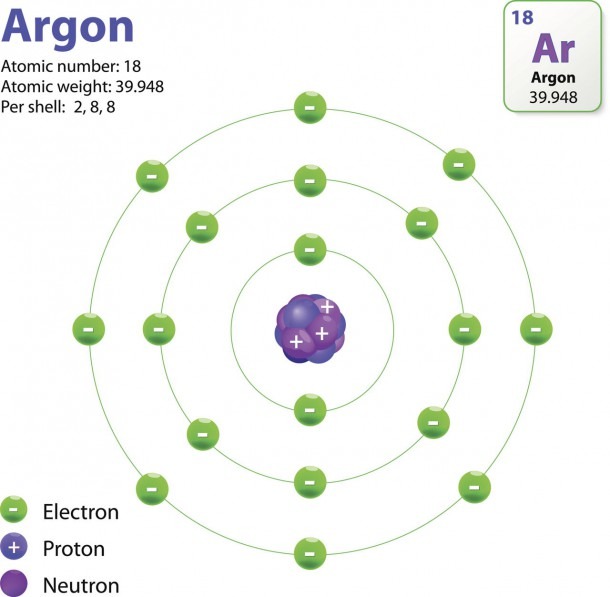
The Structure Of An Atom Explained With A Labeled Diagram Best Diagram Collection
Atomic Structure All atoms except hydrogen contain three basic subatomic particles: 1) electrons, 2) protons, and neutrons. Neutrons and protons are found at the center of the atom within a dense region called the nucleus. In contrast, electrons are found outside the nucleus in a region called the electron cloud or electron shell. 1) Electrons
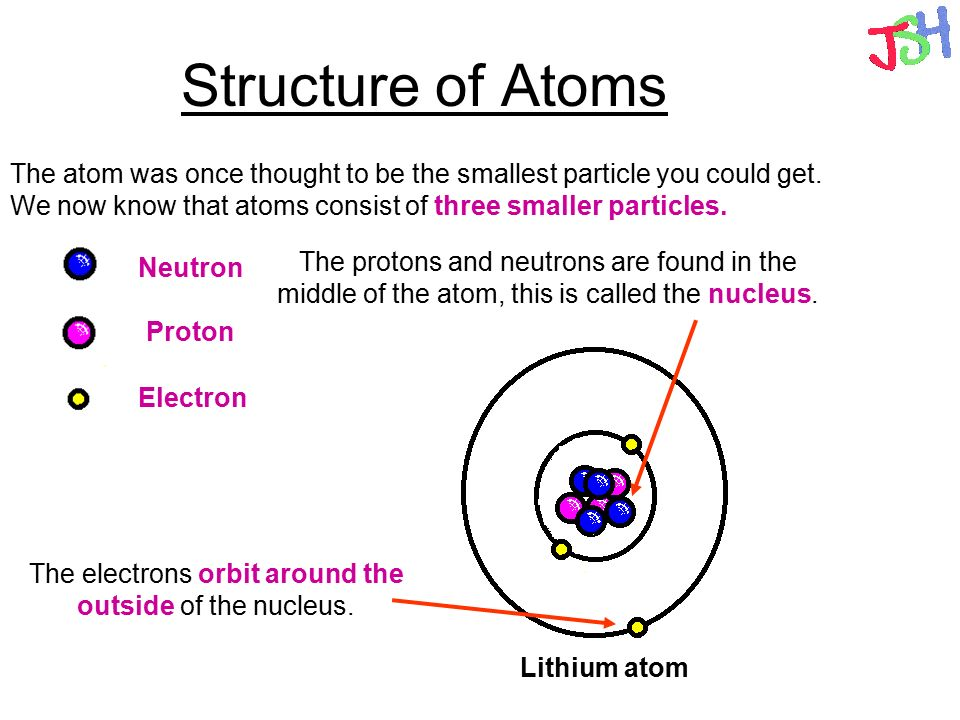
The Structure of the Atom GCSE Physics Science) AQA Revision Study Rocket
2.3 Atomic Structure and Symbolism 2.3 Atomic Structure and Symbolism Highlights Learning Objectives By the end of this section, you will be able to: Write and interpret symbols that depict the atomic number, mass number, and charge of an atom or ion Define the atomic mass unit and average atomic mass
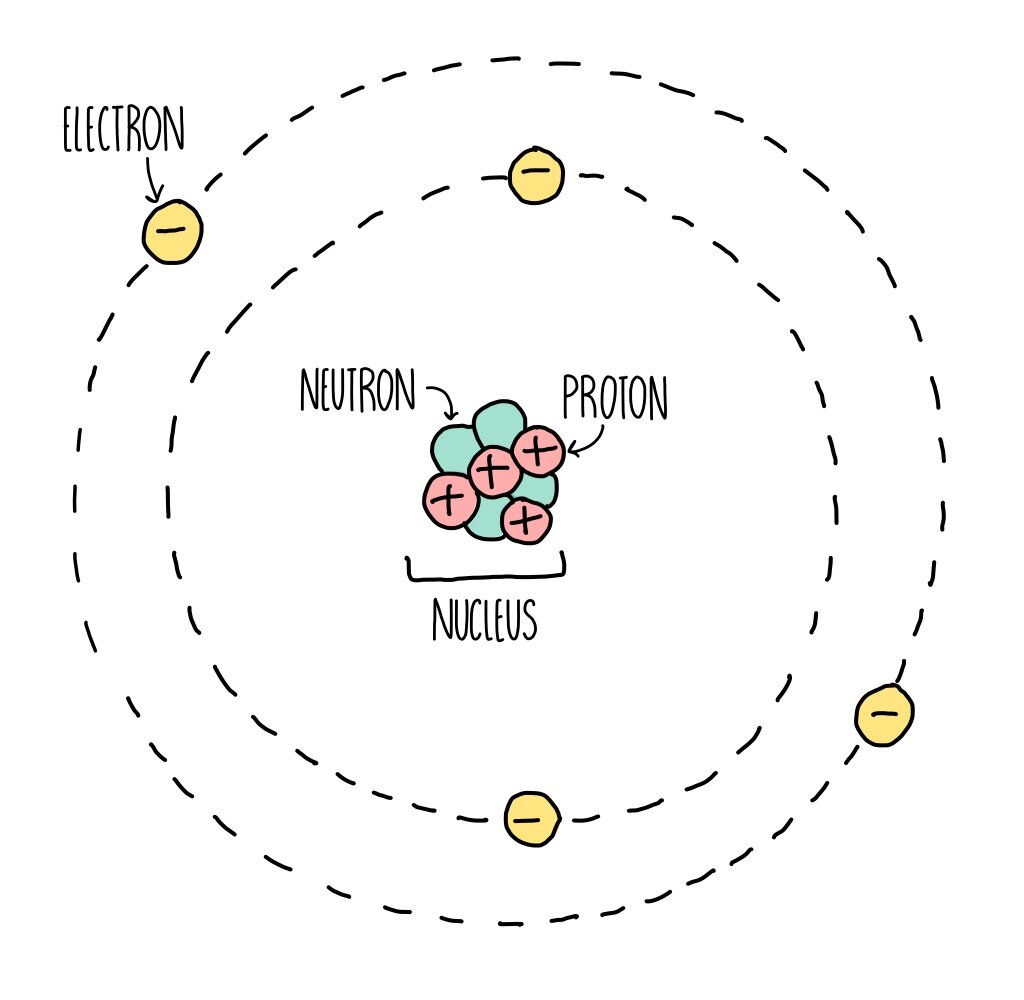
Atomic Structure (GCSE) — the science sauce
An atom is composed of two regions: the nucleus, which is in the center of the atom and contains protons and neutrons, and the outer region of the atom, which holds its electrons in orbit around the nucleus. Protons and neutrons have approximately the same mass, about 1.67 × 10 -24 grams, which scientists define as one atomic mass unit (amu.
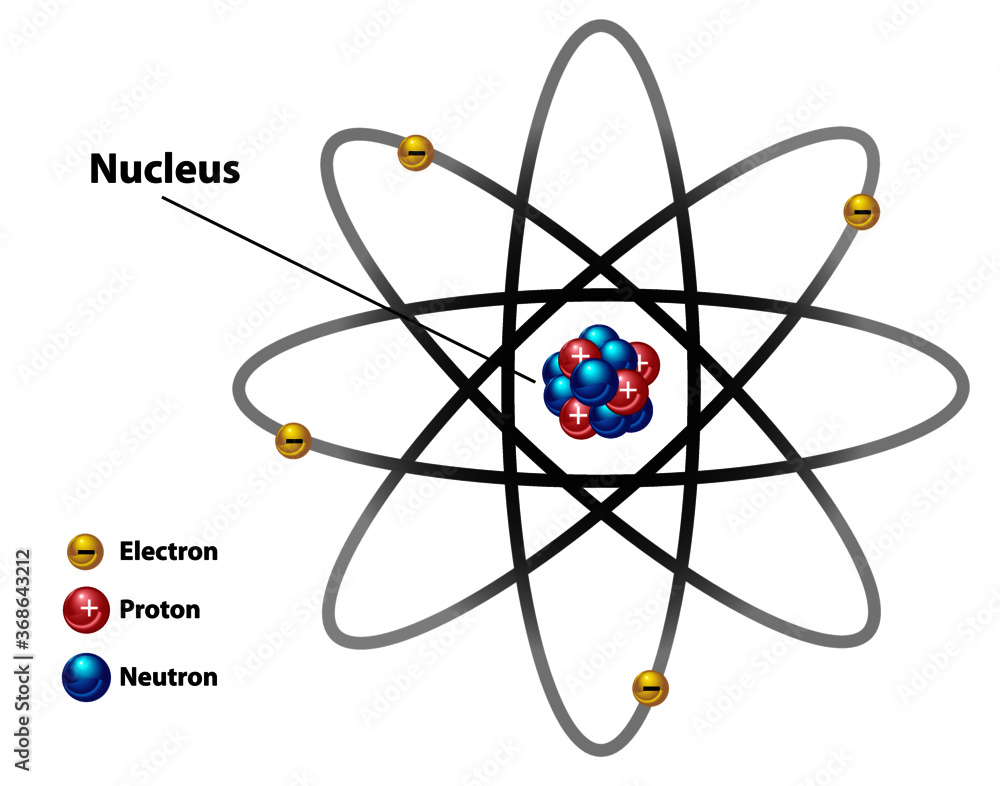
Atomic nucleus diagram labeled with electron, proton, and neutron. Stock Vector Adobe Stock
In this video we cover the structure of atoms, what are subatomic particles, energy levels, and stable and reactive atoms.Transcript and notesAtomic structur.
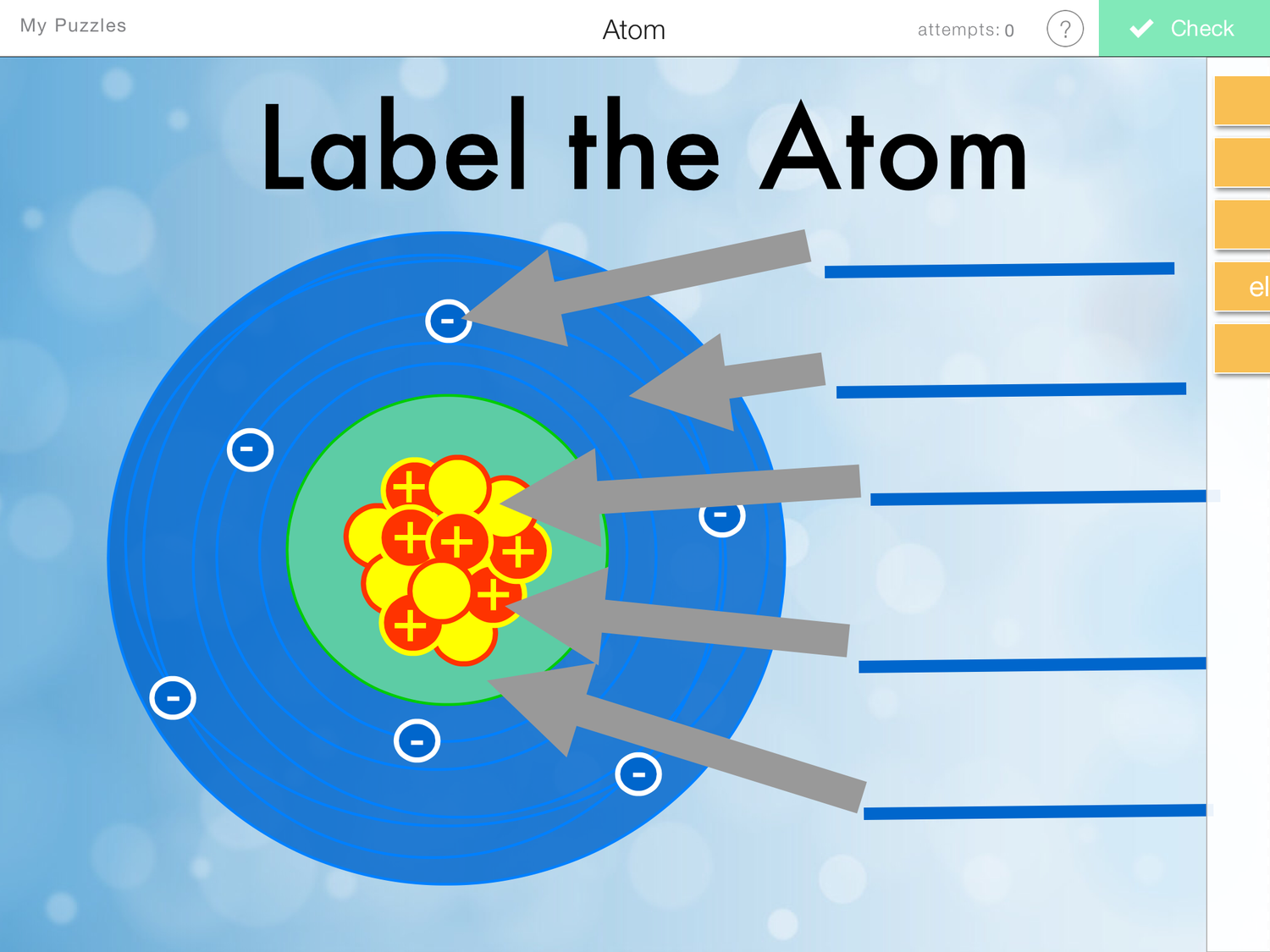
Label Parts of an Atom — Learning in Hand with Tony Vincent
The orbits are labeled by an integer, the quantum number n. Electrons can jump from one orbit to another by emitting or absorbing energy. The inset shows an electron jumping from orbit n=3 to orbit n=2, emitting a photon of red light with an energy of 1.89 eV. (more)
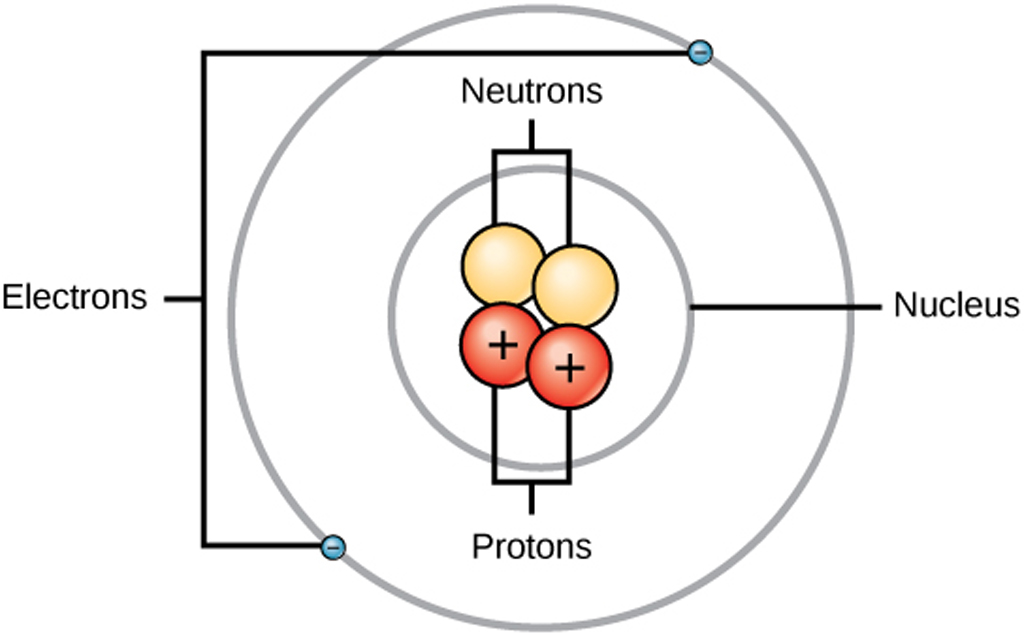
Structure of an Atom Structure & Use of Electron & Proton in Electronics
An atom is the smallest unit of matter that retains the chemical properties of an element. Atoms are able to interact with each other through bonding, to form more complex substances, also known as molecules. These interactions determine the state of matter the atoms are in, as they can be found as solids, liquids, or gases.
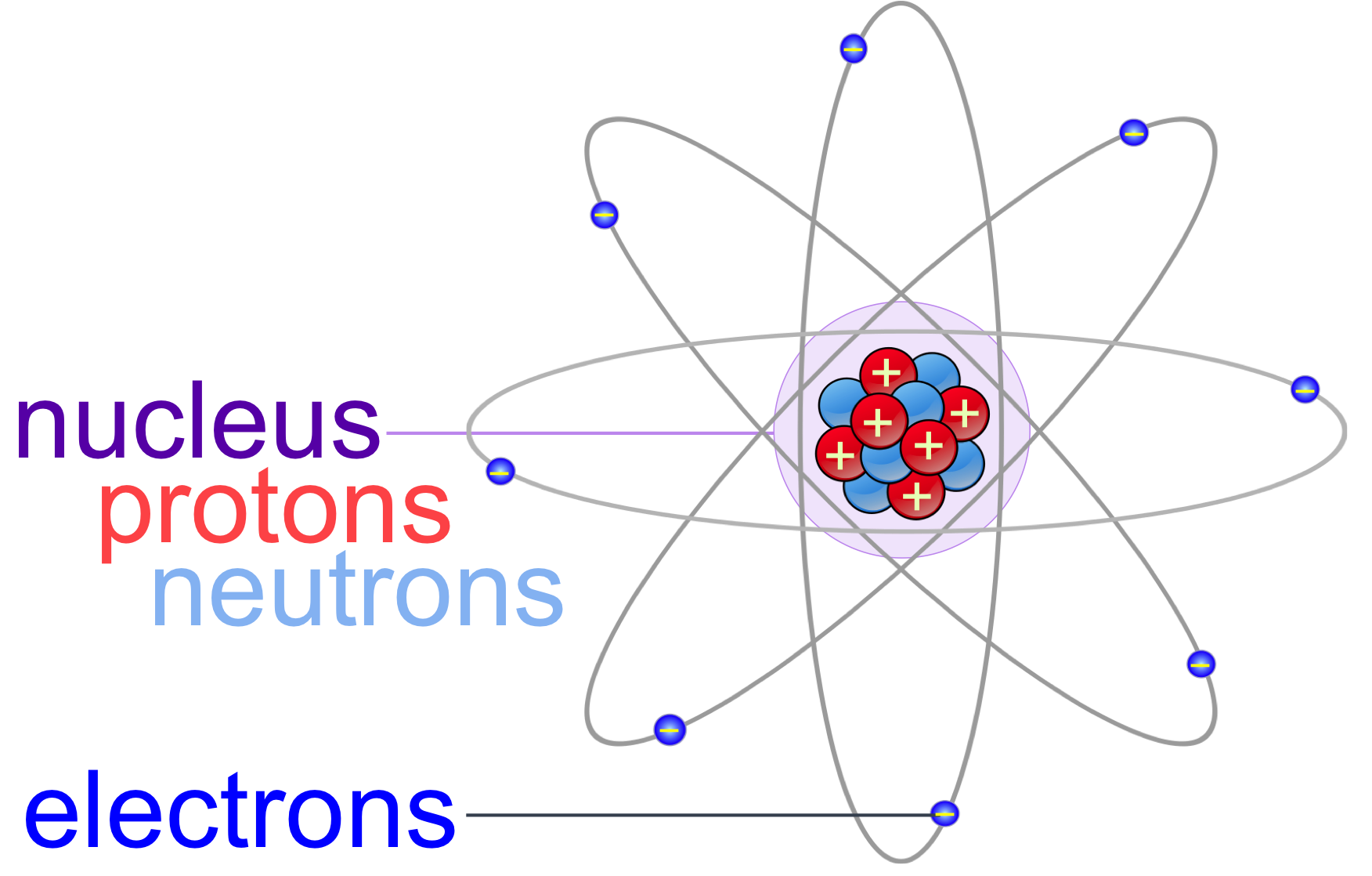
Atoms & Molecules echapter — The Biology Primer
Table 1. Properties of Subatomic Particles Charge (C) Mass (amu) Mass (g) −1.602 × 10 0.00091 × 10 The number of protons in the nucleus of an atom is its atomic number (Z). This is the defining trait of an element: Its value determines the identity of the atom.