MichaelisMentenKonstante · Einfach erklärt Enzymaktivität [mit Video] · [mit Video]

MichaelisMenten MichaelisMenten plot of MpIspS initial... Download Scientific Diagram
The Michaelis-Menten mechanism (Michaelis & Menten, 1913) is one which many enzyme mitigated reactions follow. The basic mechanism involves an enzyme ( E E, a biological catalyst) and a substrate ( S S) which must connect to form an enzyme-substrate complex ( ES E S) in order for the substrate to be degraded (or augmented) to form a product ( P.

MichaelisMentenGleichung… BASICS of ANESTHESIOLOGY boa.coach
The Michaelis-Menten mechanism (Michaelis & Menten, 1913) is one which many enzyme mitigated reactions follow. The basic mechanism involves an enzyme ( E, a biological catalyst) and a substrate ( S) which must connect to form an enzyme-substrate complex ( ES) in order for the substrate to be degraded (or augmented) to form a product ( P ).

Example of a MichaelisMenten plot (left) and a LineweaverBurk plot Download Scientific Diagram
The program determines the constants Rmax and Km of the Michaelis-Menten model using data given in Fundamentals of Chemical Reaction Engineering by M. E. Davis & R. J. Davis, McGraw Hill, 2003. This data represents the substrate (catechol) concentration versus time. This data was obtained using E. herbicola immobilized in a polymer gel to.
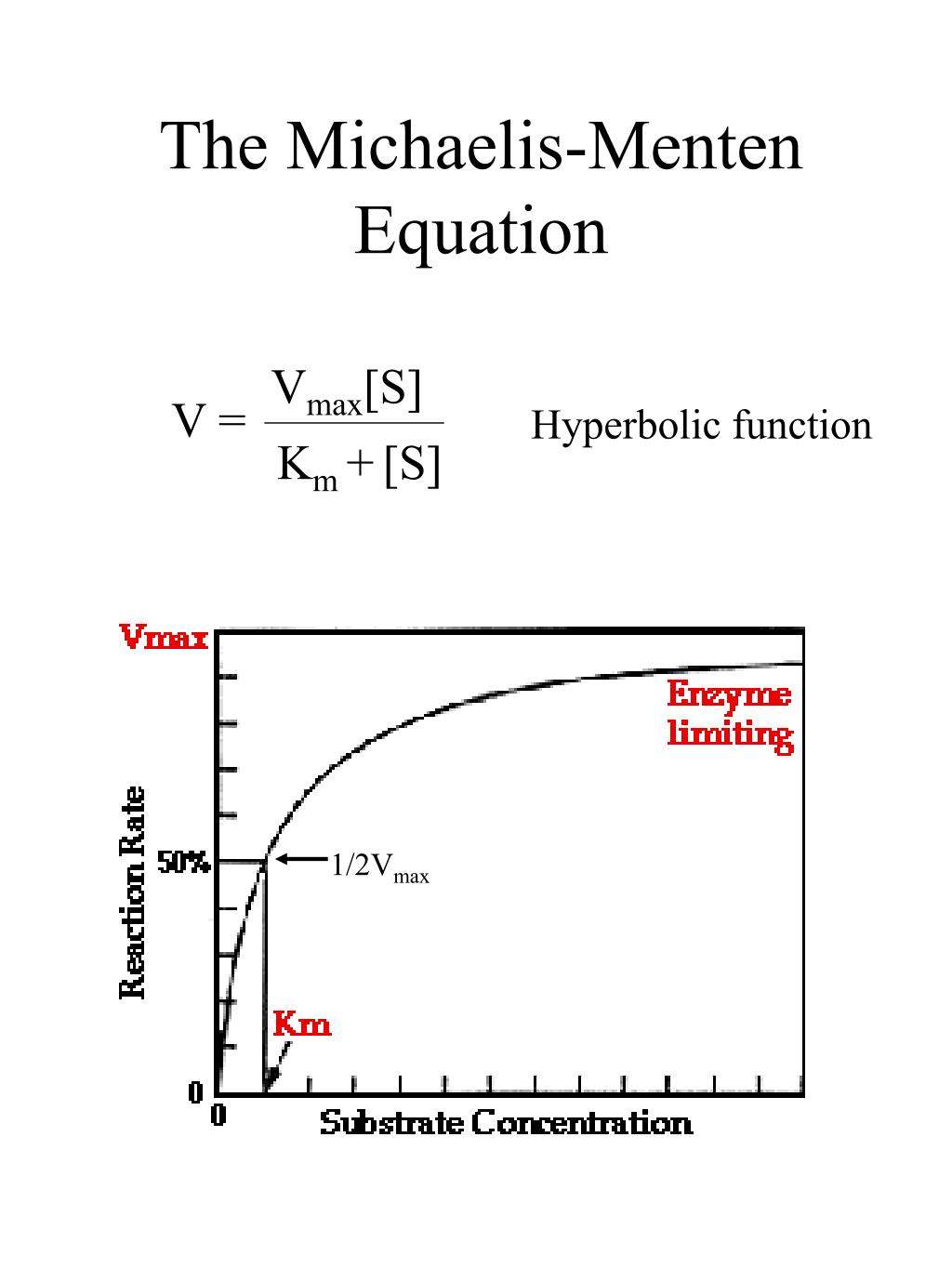
PPT LAB 3 Enzyme PowerPoint Presentation, free download ID4526880
The Michaelis-Menten equation, named after biochemist Leonor Michaelis and physician Maud Menten, "describes the relationship between the rate of substrate conversion by an enzyme (V) and the concentration of the substrate ([S])," according to the Davidson College Chemistry website. Based on this equation, the Michaelis-Menten curve can be.

2 Model MichaelisMenton plots showing predicted changes with... Download Scientific
In biochemistry, the direct linear plot is a graphical method for enzyme kinetics data following the Michaelis-Menten equation. [1] In this plot, observations are not plotted as points, but as lines in parameter space with axes and , such that each observation of a rate at substrate concentration is represented by a straight line with.

MichaelisMenten enzyme for TMT1 (green diamonds ), TMT2... Download Scientific Diagram
The Michaelis constant is the substrate concentration at which an enzyme operates at one half of its maximum velocity. How can you calculate this constant ba.

grafico MichaelisMenten Bald Mountain Science
Michaelis-Menten derivation for simple steady-state kinetics. The Michaelis-Menten equation is a mathematical model that is used to analyze simple kinetic data.The model has certain assumptions, and as long as these assumptions are correct, it will accurately model your experimental data.The derivation of the model will highlight these assumptions.
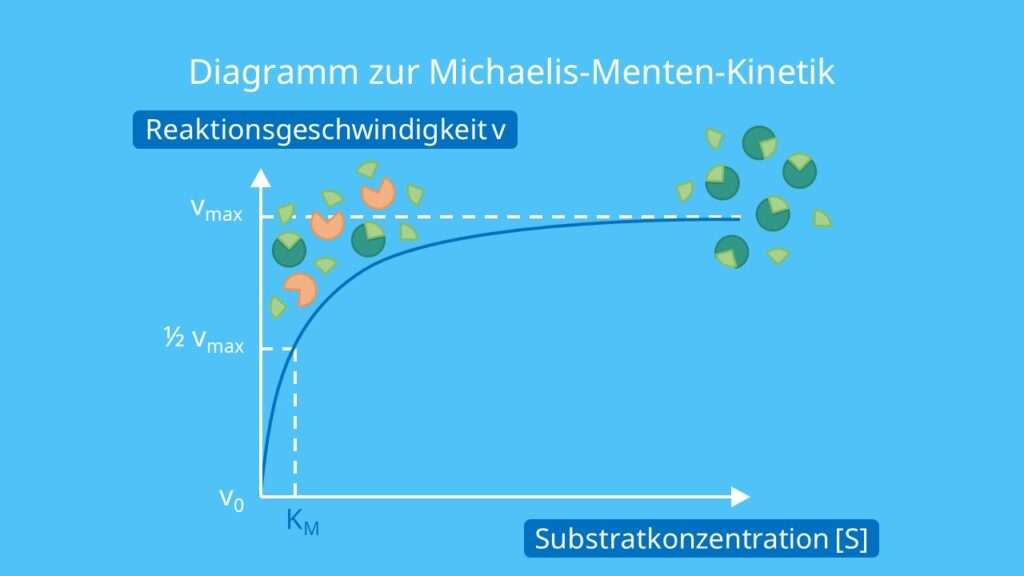
MichaelisMentenGleichung · einfach erklärt Formel [mit Video] · [mit Video]
The NLFit dialog is an interactive tool which allows you to monitor the fitting procedure during the non-linear fitting process. This tutorial fits the Michaelis-Menten function, which is a basic model in Enzyme Kinetics, and shows you some basic features of the NLFit dialog. During the fitting, we will illustrate how to perform a Global Fit.
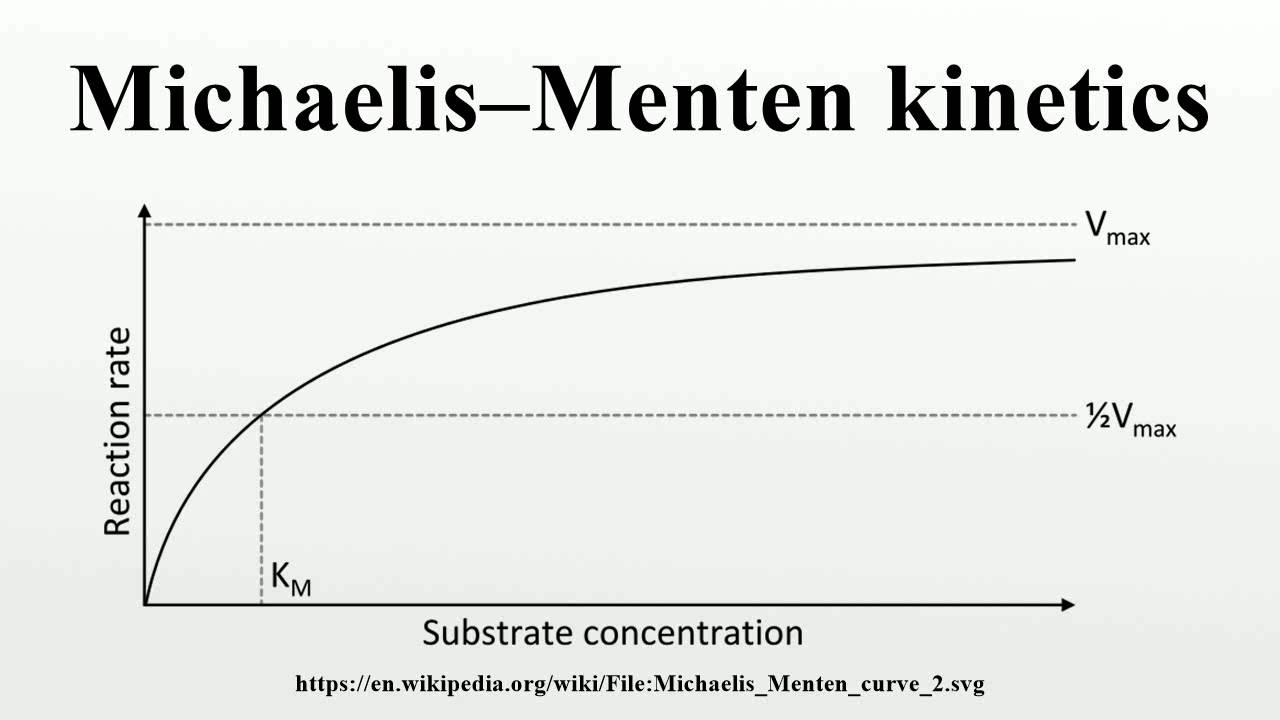
MichaelisMenten YouTube
The Michaelis-Menten equation (see below) is commonly used to study the kinetics of reaction catalysis by enzymes as well as the kinetics of transport by transporters. Typically, the rate of reaction (or reaction velocity) is experimentally measured at several substrate concentration values. The range of substrate concentrations is chosen such that very low reaction rates as well as saturating.
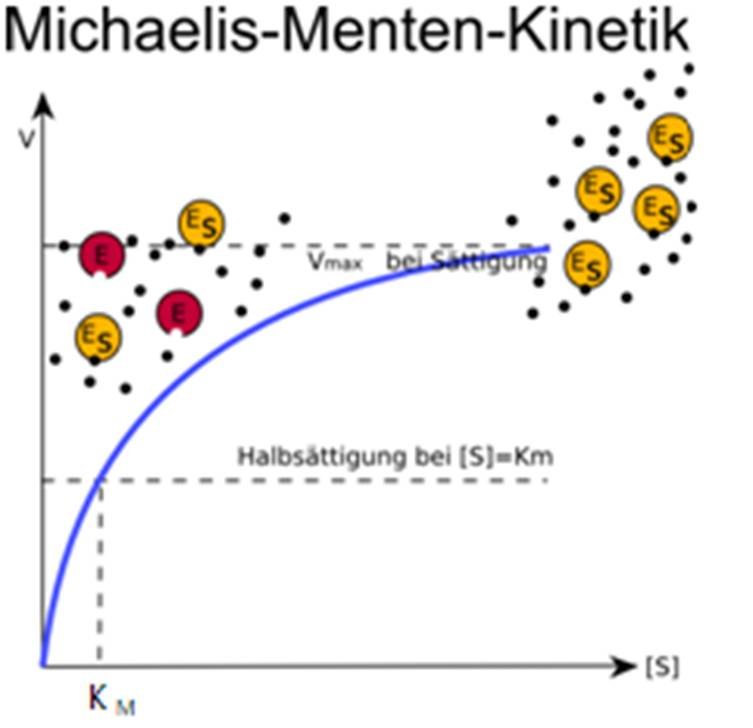
Physikalische Chemie
Michaelis Menten function to describe relation of concentration of substrate and enzyme velocity. Sample Curve Parameters. Number: 2 Names: Vmax, Km Meanings: Vmax = Maximum Velocity, Km = Michaelis Constant Lower Bounds: Vmax > 0.0, Km > 0.0 Upper Bounds: none Script Access nlf_MichaelisMenten (x,Vmax,Km) Function File. FITFUNC\MichaelisMenten.FDF

The Michaelis Menten plot for HRP to determine K m and V max of the... Download Scientific
The reaction is zero-order kinetics. Figure 5.3.2: Diagram of reaction velocity and Michaelis-Menten kinetics. v = Vmax 2 = Vmax[S] Km + [S] (5.3.17) (5.3.17) v = V m a x 2 = V m a x [ S] K m + [ S] Therefore, Km K m is equal to the concentration of the substrate when the rate is half of the maximum velocity.

Developing a three‐dimensional animation for deeper molecular understanding of michaelismenten
Step 4: Format the Scatter Plot. Next, format the scatter plot to turn it into a Michaelis-Menten plot. First, remove the vertical axis and format the horizontal axis to become a logarithmic scale. This can be done by right-clicking the horizontal axis and selecting "Format Axis.". Then, select "Logarithmic Scale" under the "Scale.

The MichaelisMenten Enzyme Model
Page ID. Two 20 th century scientists, Leonor Michaelis and Maud Leonora Menten, proposed the model known as Michaelis-Menten Kinetics to account for enzymatic dynamics. The model serves to explain how an enzyme can cause kinetic rate enhancement of a reaction and explains how reaction rates depends on the concentration of enzyme and substrate.

MichaelisMenten study of the nonenzymatic RNA copying rates of 2AIpG... Download Scientific
In this article we will discuss about the Michaelis-Menten Constant and Significance of Michaelis-Menten Constant.. Michaelis-Menten Constant: In an enzyme catalysed reaction when there is large excess of substrate and the enzyme concentration is held constant, if substrate concentration (S) is plotted against velocity (V) or reaction rate, a hyperbolic curve is obtained (fig. 10.13).

The MichaelisMenten diagram of soluble and immobilized form of DHODH... Download Scientific
Michaelis-Menten kinetics. In biochemistry, Michaelis-Menten kinetics, named after Leonor Michaelis and Maud Menten, is the simplest case of enzyme kinetics, applied to enzyme-catalysed reactions of one substrate and one product. It takes the form of an equation describing the reaction rate (rate of formation of product P, with.

Illustration of MichaelisMenten function. Download Scientific Diagram
Named after Leonor Michaelis and Maud Menten, the Michaelis-Menten kinetics is the most basic form of enzyme kinetics. The equation is given by: \ ( v = \frac {d [p]} {dt} = \frac {Va} {K_m + a} \) Advertisements. In this equation, V or often represented as Vmax , signifies the maximum rate achieved by the system when the substrate.