Phosphorus Definition, Facts, Symbol, Discovery, Property, Uses

PO4 3 Lewis Structure The Phosphate Ion YouTube
Example 8.3. 1: Benzene. Benzene is a common organic solvent that was previously used in gasoline; it is no longer used for this purpose, however, because it is now known to be a carcinogen. The benzene molecule ( C 6 H 6) consists of a regular hexagon of carbon atoms, each of which is also bonded to a hydrogen atom.
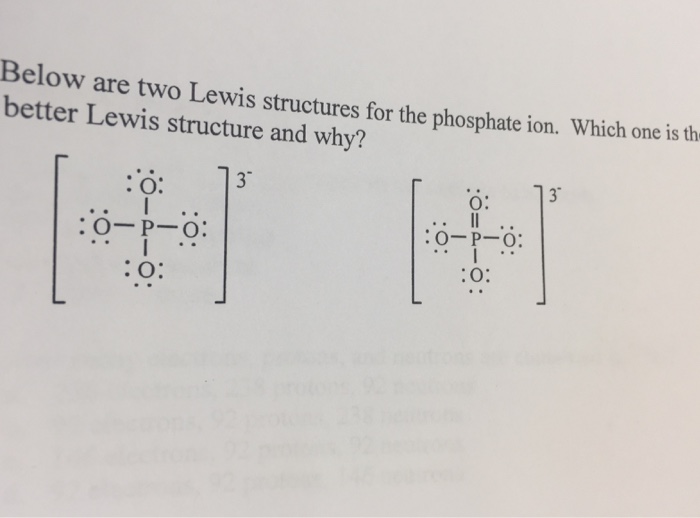
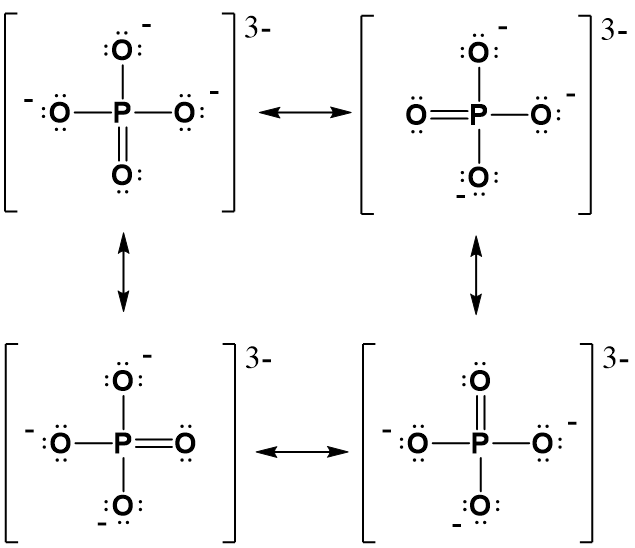
Solved Below are two Lewis structures for the phosphate ion.
How to Draw Lewis Structure of PO4 3- I Easy & Quick Science Genius 26 subscribers Subscribe 0 2 views 9 months ago This video will explain how to draw a skeletal structure of phosphate.

Simple Method for writing Lewis Structures of the phosphate ion(PO4)3
1 Answer Sorted by: 6 Calcium phosphate crystallises in a number of different modifications of which β- CaX3(POX4)X2 C a X 3 ( P O X 4) X 2 is the stable room temperature and ambient pressure modification. It has a rather complex structure not easily described, consisting of chains and rings with phosphate and calcium ions in specific patterns.

Phosphorus Lewis Dot Structure Drawing, Several Compounds And Detailed
Steps of drawing PO4 3- lewis structure Step 1: Find the total valence electrons in PO4 3- ion. In order to find the total valence electrons in PO4 3- ion (phosphate ion), first of all you should know the valence electrons present in phosphorus atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)
blood calcium hormone feedback loop
This chemistry video tutorial explains how to draw the lewis structure of PO4 3-, the phosphate ion. It also discusses the formal charge and resonance struc.
PO4 3 Lewis Structure How to Draw the Lewis Structure for PO43 YouTube
A step by step guide to drawing the lewis structure of PO4, phosphate. There is a good bit of detail here showing how you count valence electrons, draw the.
Phosphate Anion Chemical Structure Photograph by Molekuul/science Photo
Phosphate is a very weak oxidizing agent. Since the phosphorus is in its highest oxidation state in phosphate ion, this ion cannot act as a reducing agent. This page titled Phosphate Ion (PO₄³⁻) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by James P. Birk. Phosphate ion is a reasonably strong base.
CH105 Chapter 5 Introduction to Organic Chemistry Chemistry
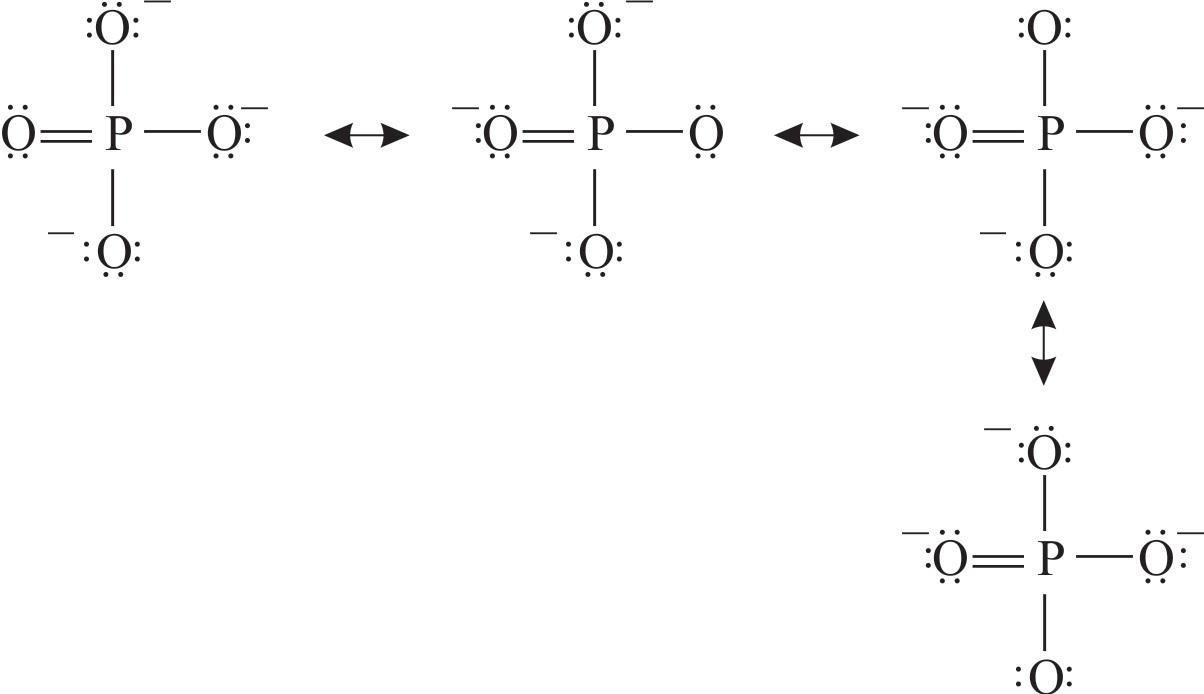
1.2.2 Lewis Structures of Polyatomic Molecules or Ions.. Figure 1.2m phosphate anion Lewis structure. Elements in Period 3 (or higher) have 3 (or more than 3) principle shells, so the d orbital is available in the valence shell. That is why they can accommodate more than 8 electrons.
:max_bytes(150000):strip_icc()/phosphate-anion-58c046973df78c353ca2b689.jpg)
List of Common Polyatomic Ions
To draw the Lewis structure of any compound, we always use the concept of valence electrons (number of electrons in the outer shell of each atom). The overall charge on the phosphate ion is -3 and the phosphorus atom is in an oxidation state of +5.
Identify The Lewis Structure Resonance Forms For Po That My XXX Hot Girl
In lewis structure, there should be charges on atoms. Phosphate ion | PO 43- Phosphate ion is one of the oxyanion of phosphorous. Phosphorous is at +5 oxidation state in PO 43-. Also, phosphate ion has a -3 charge. Lewis structure of PO 43- ion In the lewis structure of PO 43-, three is a double bond between phosphorous atom and one oxygen atom.
41. What is the Lewis dot structure of phosphate ion? how many
The Lewis symbol is the chemical symbol of an element with valence electrons represented as dots. The Lewis symbols of some elements are shown here: Figure 1.2a The Lewis structures of aluminum, tin, nitrogen, chlorine and bromine. For simple diatomic molecules, combining the Lewis symbols of each element gives its Lewis structure.

Phosphate
When drawing Lewis dot structures, the overall charge on a polyatomic ion is equal to the sum of the formal charges on each atom in the ion.. phosphate is a polyatomic ion that always has a charge of 3-. Since the overall charge for an ionic compound is zero, we can use the chemical formula and the charge on phosphate to calculate the charge.
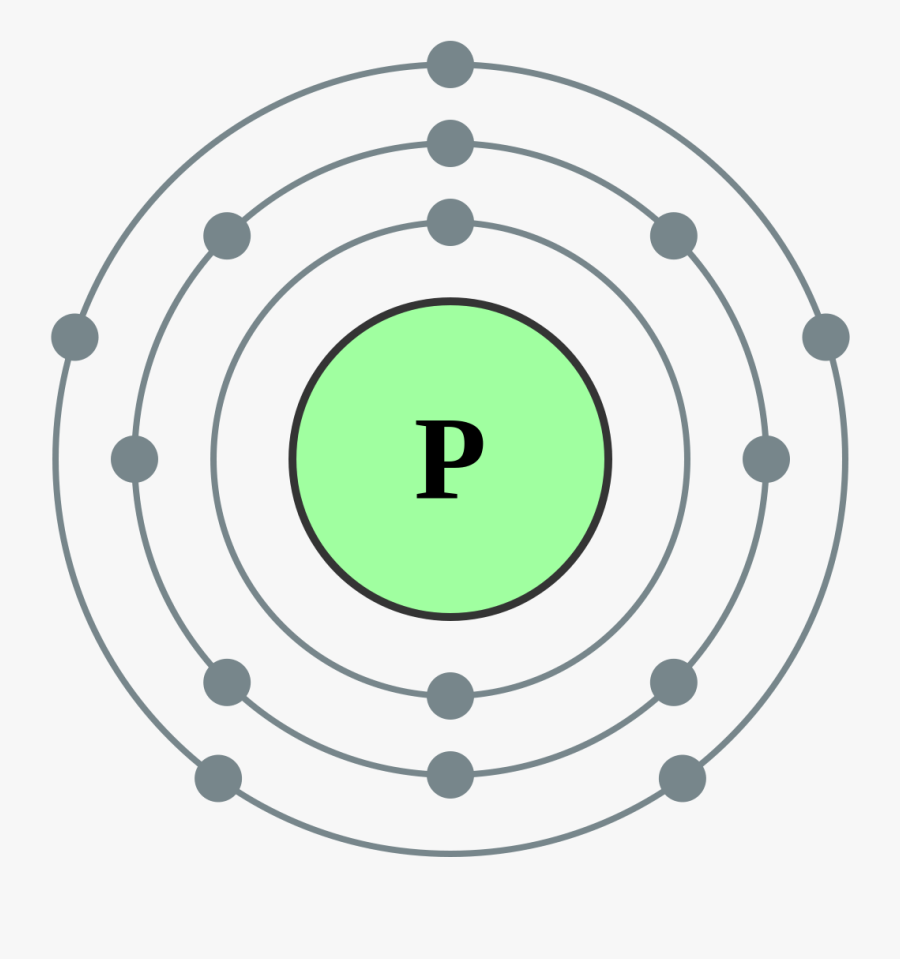
Electron Shell 015 Phosphorus Phosphorus Electron Dot Diagram , Free
Share 994 views 10 years ago This is a tutorial on how to draw a Lewis dot structure (diagram) of a phosphate ion.more.more This is a tutorial on how to draw a Lewis dot structure.

Lewis Dot Structure of Phosphate (PO4 3).....No More Confusion
A single phosphate is linked to two organic groups is called phosphate diester. The backbone of DNA is linked by phosphate diesters. Organic phosphates are often abbreviated using ' OP O P ' and ' OPP O P P ' for mono- and diphosphates, respectively. For example, glucose-6-phosphate and isopentenyl diphosphate are often depicted as shown below.
Lewis Dot Diagram For Phosphorus
This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
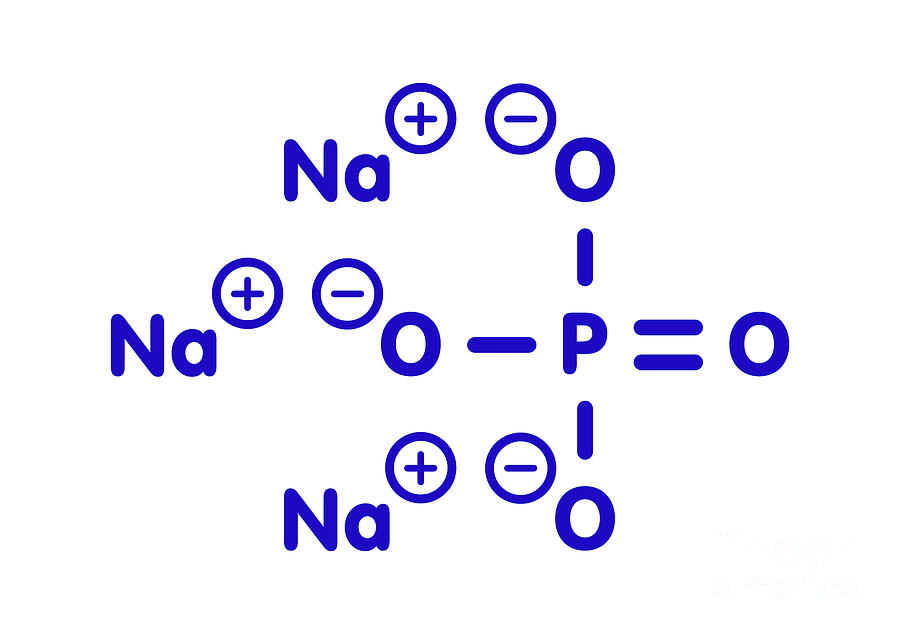
Sodium Phosphate Chemical Structure Photograph by Molekuul/science
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".