A Look At The Ethanol Lies
Ethanol Molecule Alcohol Universe Chemistry PNG, Clipart, Alcohol
Lewis structure is a representation of all the bonds and lone pairs of different atoms that a compound has. This is a 2-D representation and it helps us to understand more about the properties of the compound. Let's move step-by-step and see how the Lewis Structure of C2H5OH can be made. Step 1: Finding the valence electrons for each atom.

Ethanol Formula Formula, Composition, Uses, Structure Embibe
Subscribed 9.6K views 2 years ago An explanation of the molecular geometry for the C2H5OH (Ethanol) including a description of the C2H5OH bond angles..more.more An explanation of the.

Structural chemical formula and model of ethanol Vector Image
Lewis Dot Structure of CH3CH2OH (Ethanol) kentchemistry.com 25.1K subscribers Subscribe Subscribed 80K views 12 years ago Every Video I quickly take you through how to draw the Lewis Structure.

Ethanol Formula Formula, Composition, Uses, Structure Embibe
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
Ethanol Metabolism Concise Medical Knowledge
Lewis structure of C2H5OH (or Ethanol) contains five C-H bonds, one O-H bond and one C-O bond. The two Carbon atoms (C) are at the center and it is surrounded by Hydrogen atoms (H) and one OH group. The oxygen atom has 2 lone pairs. Let's draw and understand this lewis dot structure step by step. (Note: Take a pen and paper with you and try.

Lewis Structure For Ethanol
Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

Schema De Lewis De Ethanol
The Lewis structure of ethanol helps us understand its physical and chemical properties, including its molecular geometry, bond angles, polarity, and reactivity. The molecular geometry of ethanol is tetrahedral, with the oxygen atom at the center and the carbon and hydrogen atoms arranged around it. This geometry is a result of the.
A Look At The Ethanol Lies
An alcohol is an organic compound with a hydroxyl (OH) functional group on an aliphatic carbon atom. Because OH is the functional group of all alcohols, we often represent alcohols by the general formula ROH, where R is an alkyl group.Alcohols are common in nature. Most people are familiar with ethyl alcohol (ethanol), the active ingredient in alcoholic beverages, but this compound is only one.
C2h5oh ethanol molecule Royalty Free Vector Image
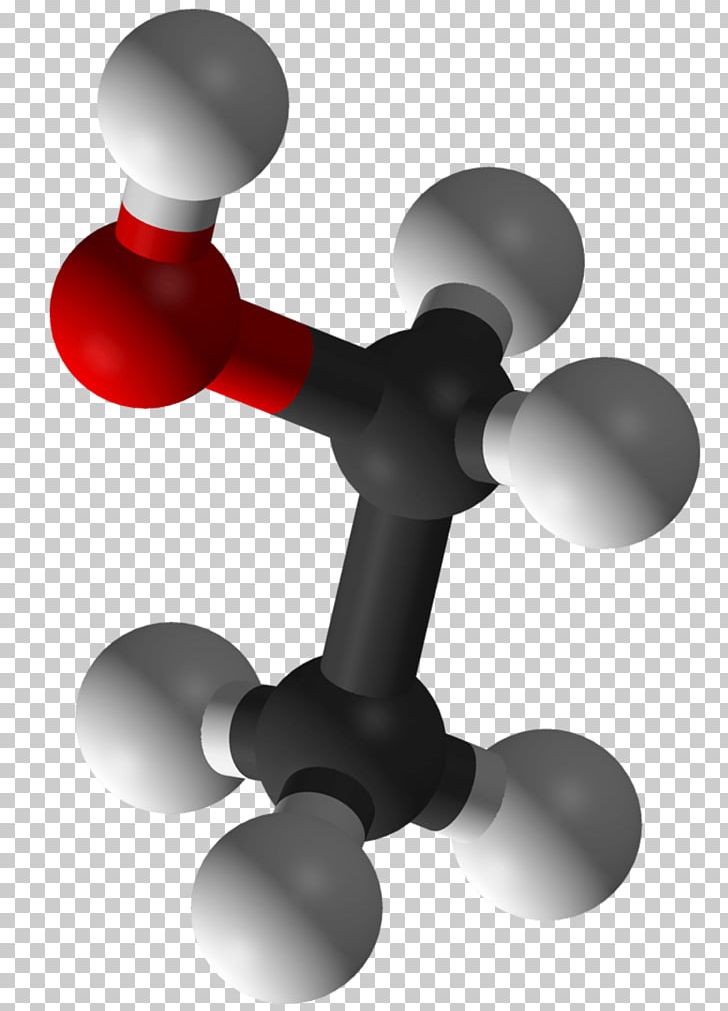
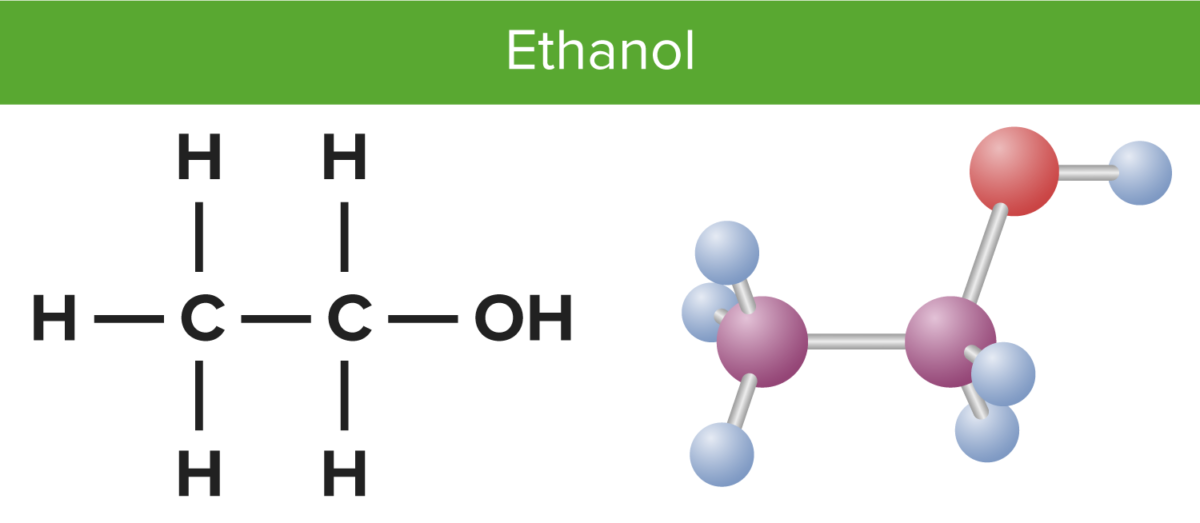
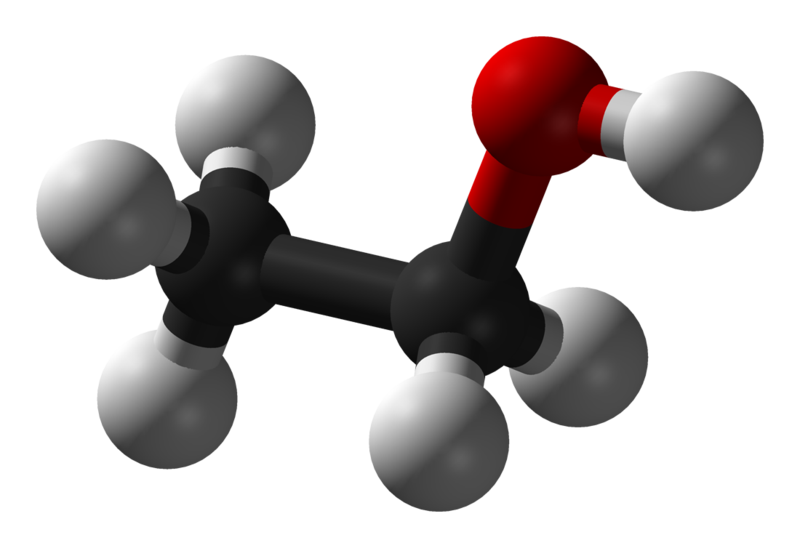
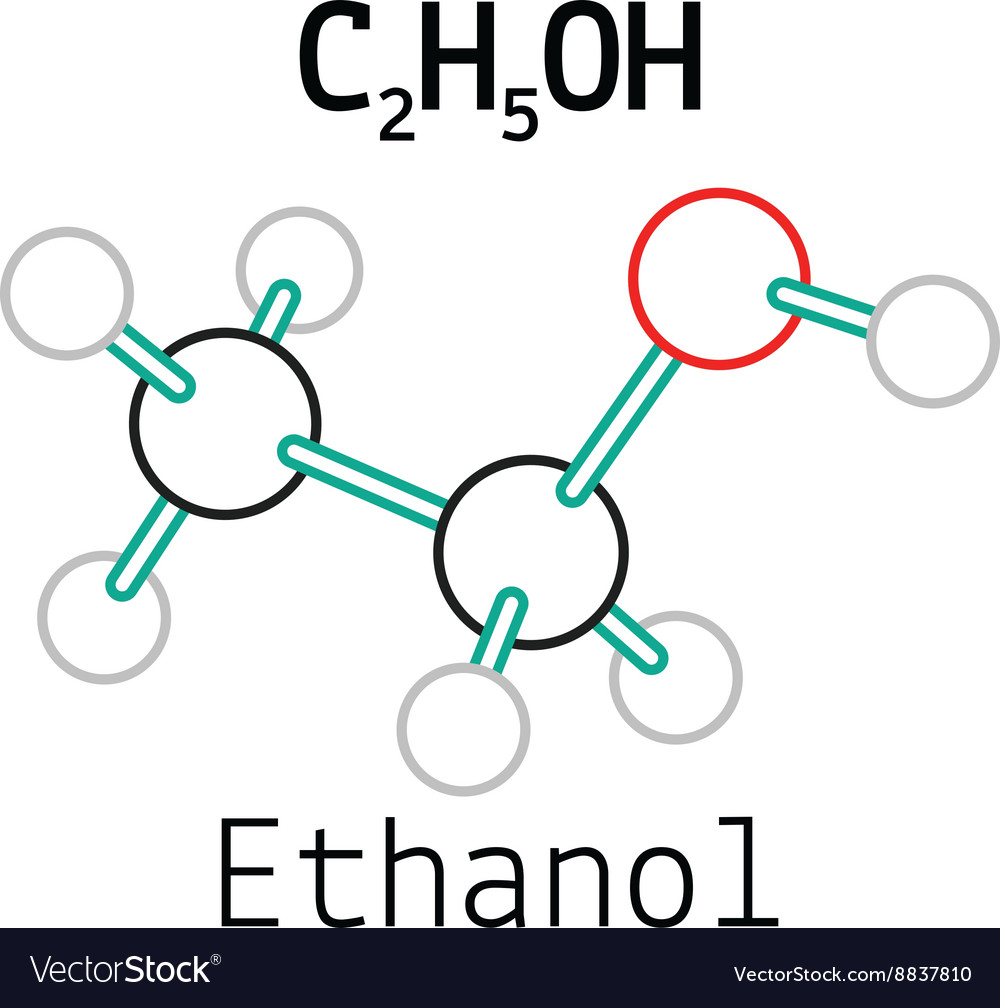
Its molecular formula is C2H5OH, and it consists of two carbon atoms, six hydrogen atoms, and one oxygen atom. The Lewis dot structure of ethanol shows the arrangement of these atoms and their valence electrons.
/GettyImages-136810090-56a133b25f9b58b7d0bcfd93.jpg)
Ethanol Molecular Formula and Empirical Formula
In the lewis structure of ethanol, all bonds between atoms are single bonds. One hydrogen atom has joint with oxygen atom and that oxygen atom is joint with one carbon atom. There are two lone pairs in the valence shell of oxygen atom. Steps of drawing lewis structure of CH 3 CH 2 OH

Ethanol Formula Composition, Uses, Structure, Density
Ethanol and its History. In the mid 1800's the use of grain alcohol (ethanol) increased as a major source of lamp fuel. Gas Lamp. Unfortunately, there were taxes placed on alcohol to help fund the.
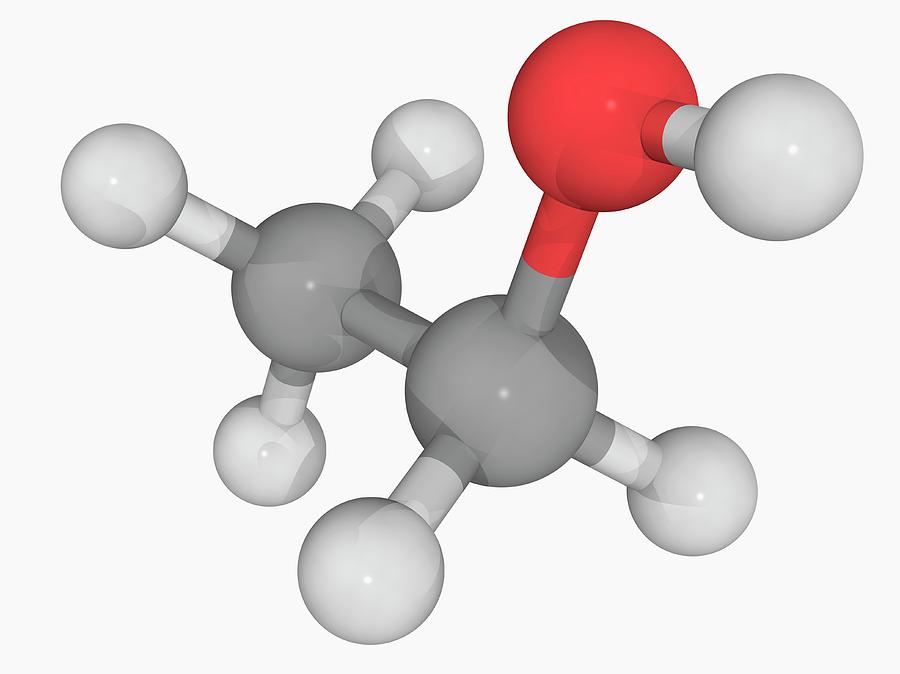
Ethanol Molecule Photograph by Laguna Design/science Photo Library Pixels
How to Draw a Lewis Structure for Ethanol? C2H6OLewis Structure: https://www.youtube.com/watch?v=4rRVPeeZRmc&list=PLDwv-O7TJyNjAB0ak6We0sQ8t_a7D2cJ7Subscribe.
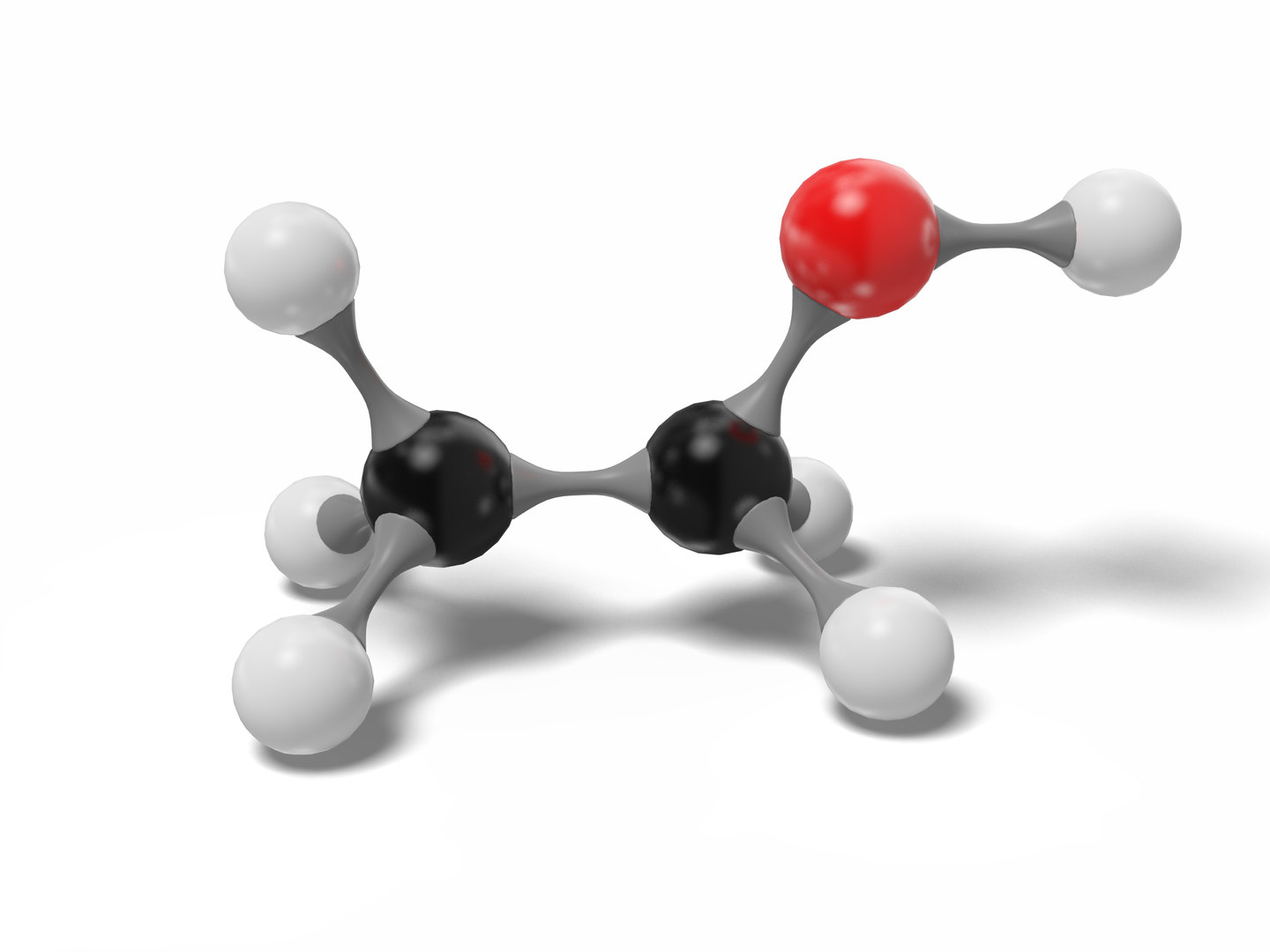
Ethanol molecule c2h6o modeled 3D model TurboSquid 1543644
Steps of drawing C2H5OH lewis structure Step 1: Find the total valence electrons in C2H5OH molecule. In order to find the total valence electrons in a C2H5OH molecule, first of all you should know the valence electrons present in carbon atom, hydrogen atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)

Is Ethanol (C2H5OH) Polar or Nonpolar? Techiescientist
Ethanol Lewis structure November 2, 2023 by Deep The information on this page is fact-checked. Ethanol Lewis structure CH 3 CH 2 OH or C 2 H 5 OH or C 2 H 6 O (ethanol) has two carbon atoms, six hydrogen atoms, and one oxygen atom. In the ethanol Lewis structure, there are five C — H bonds, one C — C bond, one C — O bond, and one O — H bond.
Lewis Structure For Ethanol
C2H5OH or Ethanol is an organic chemical compound, which can also be represented as CH3-CH2-OH. Ethanol is a colourless liquid with a distinct odour and has a pungent taste. It has flammable properties; and gives a blue colour flame when burnt.

Éthanal Définition et Explications
A step-by-step explanation of how to draw the C2H5OH Lewis Dot Structure (Ethanol (Ethyl alcohol)).For the C2H5OH structure use the periodic table to find th.