H2O Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar

Draw The Lewis Structure Of H2o Fotodtp
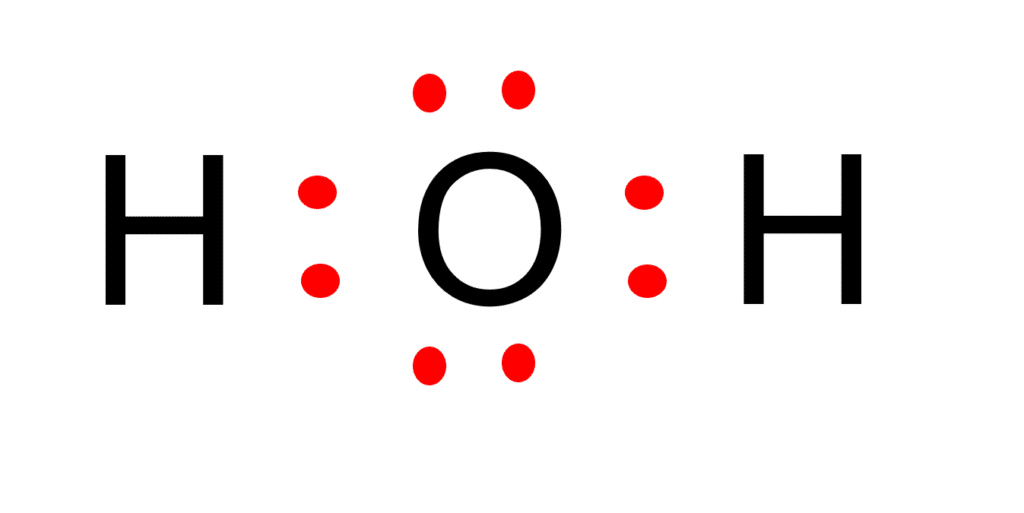
We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols: The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen.
Lewis Structures Hydrogen (H2), and Water (H2O) What's Insight
Structure of Water. Water is a simple molecule consisting of one oxygen atom bonded to two different hydrogen atoms. Because of the higher electronegativity of the oxygen atom, the bonds are polar covalent ( polar bonds ). The oxygen atom attracts the shared electrons of the covalent bonds to a significantly greater extent than the hydrogen atoms.

【4 Steps】H2O Lewis StructureLewis Structure for H2O (Water)Lewis Dot Structure for Water(H2O)
Lewis Dot Structure of H2O, (Water) kentchemistry.com 25.1K subscribers Subscribed 162K views 12 years ago Every Video I quickly take you through how to draw the Lewis Structure of water,.

【4 Steps】H2O Lewis StructureLewis Structure for H2O (Water)Lewis Dot Structure for Water(H2O)
A step-by-step explanation of how to draw the H2O Lewis Dot Structure (Water).For the H2O structure use the periodic table to find the total number of valenc.

H2O Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
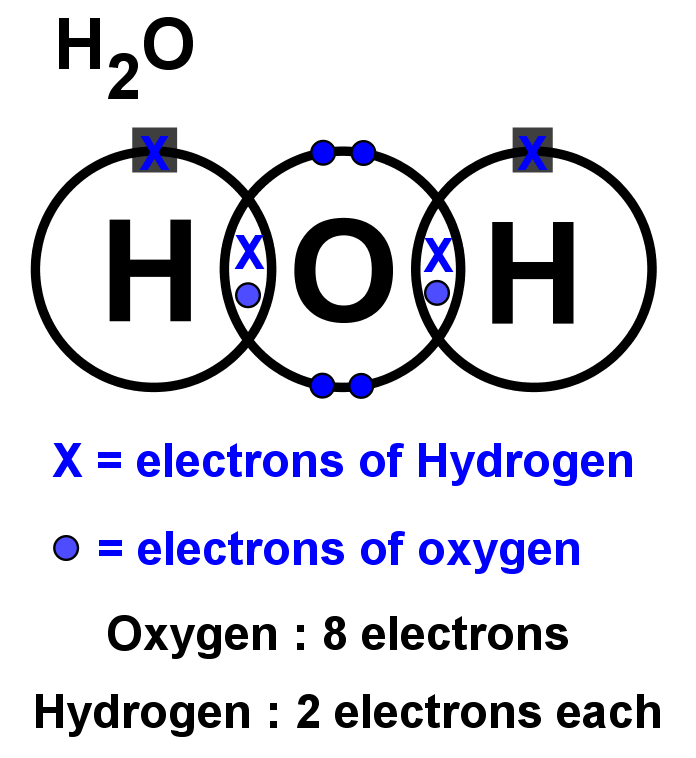
The Lewis structure of hydrogen and 2 oxygen atoms shows a total of eight valence electrons participate in the bond formation to form a single triatomic H2O molecule. Here, we need to understand how the Lewis structure is drawn for the H2O molecule: Look for the total valence electrons: It is eight to form a single H2O molecule.
What is the molecular geometry of "H"_2"O"? Draw its VSEPR structure. Socratic
We can illustrate the formation of a water molecule from two hydrogen atoms and an oxygen atom using Lewis dot symbols: The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen.
Lewis structures StudyPug
Step #1: Calculate the total number of valence electrons. Here, the given molecule is H2O (water). In order to draw the lewis structure of H2O, first of all you have to find the total number of valence electrons present in the H2O molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).
Lewis Dot Diagram For H2o Free Diagram For Student
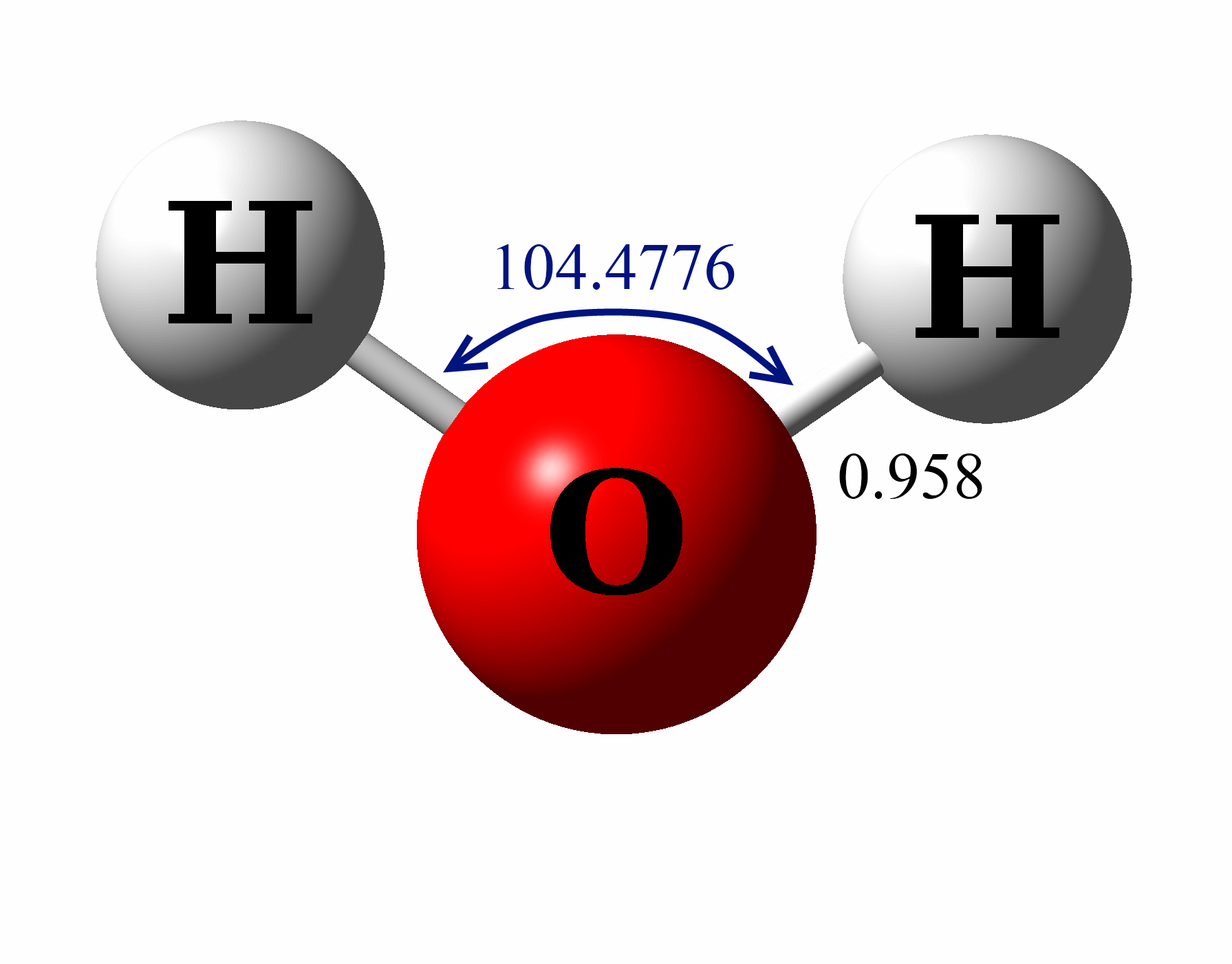
The Lewis Structure for water is useful because it allows to determine the molecular geometry and the polarity of the molecule. Because of the two lone pairs, H 2 O will have a bent molecular geometry and it will be a polar molecule. Remember that Hydrogen only needs two electrons to have a full outer shell. Video: Drawing the Lewis Structure.

H2O Lewis structure and Molecular Geometry [No1 Best Explanation] Science Education and Tutorials
A step-by-step explanation of how to draw the H2O Lewis Dot Structure (Water).For the H2O structure use the periodic table to find the total number of valenc.

H2O Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
First, we need to draw the Lewis structure of H 2 O. In short, these are the steps you need to follow for drawing a Lewis structure: 1. Write the correct skeletal structure for the molecule. * Hydrogen atoms are always terminal (only one bond) * Put more electronegative elements in terminal positions 2. Sum the valence electrons from all the atoms.

H2O Lewis StructureHow to draw H2O Lewis Structure (Water)H2O Electron Dot Structure for
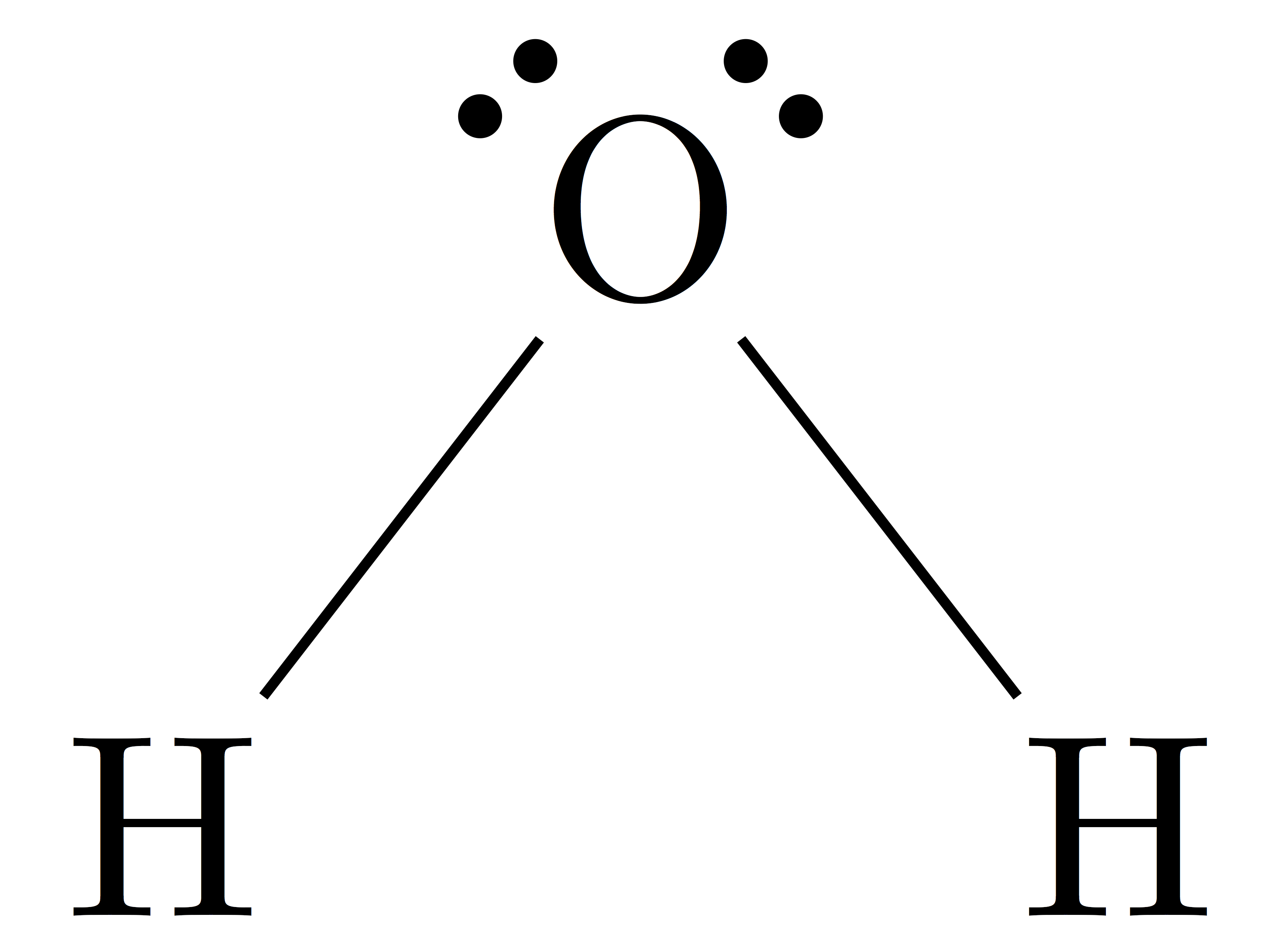
The Lewis structure of H₂O is Answer link You can find a procedure for drawing Lewis structures at this location. For H₂O, O must be the central atom The skeleton structure is H-O-H. O has 6 valence electrons, and each H has one. You must arrange 8 electrons in pairs so that O has 8 and each H has two electrons in its valence shell.

Lewis Structure Water Molecule Vector Illustration Stock Vector (Royalty Free) 2215045509
Lewis Structure of H2O Water, one of the Earth's major constituents, has the molecular formula H 2 O. A molecule of water is made up of two hydrogen atoms and one oxygen atom that are joined together by a covalent bond. Furthermore, two or more H 2 O molecules join together by hydrogen bonds to form a compound.

estructura lewis de h2o Brainly.lat
Share Save 60K views 4 years ago A quick explanation of the molecular geometry of H2O (Water) including a description of the H2O bond angles..more.more Molecular Geometry: Rules,.

H2O Lewis structure and Molecular Geometry [No1 Best Explanation] Science Education and Tutorials
1) Elementary models: The Lewis structure predicts that two lone pairs are (a) localized on the oxygen atom of water and that (b) both lone pairs are equivalent. The Lewis structure, combined with Valence Bond Theory, would predict that lone pairs occupy two equivalent hybridized \(sp^3\) atomic orbitals on oxygen.

H2O Molecular Geometry / Shape and Bond Angle (precise angle is 104.45) YouTube
H 2 O lewis structure In the lewis structure of H 2 O, there are two single bonds around oxygen atom. Hydrogen atoms are joint to oxygen atom through single bonds. Also, there are two lone pairs on oxygen atom. Water molecule is a simple molecule. Drawing lewis structure of water molecule is simple than some of other complex molecules or ions.

H2O Lewis structure and Molecular Geometry [No1 Best Explanation] Science Education and Tutorials
In the Lewis structure of H2O structure there are a total of 8 valence electrons. H2O is also called Water. ---- Steps to Write Lewis Structure for compounds like H2O ---- 1. Find the.