【5 Steps】Oxygen Electron Configuration in Just 5 Steps Electron Configuration of Oxygen(O)

【5 Steps】Oxygen Electron Configuration in Just 5 Steps Electron Configuration of Oxygen(O)
The first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2 s orbital (Figure 8.3. 3 or 8.3. 4 ). Thus, the electron configuration and orbital diagram of lithium are:

Oxygen Atom Science Notes and Projects
The electron configuration states where electrons are likely to be in an atom. If you don't have a chart, you can still find the electron configuration. Use the element blocks of the periodic table to find the highest electron orbital.
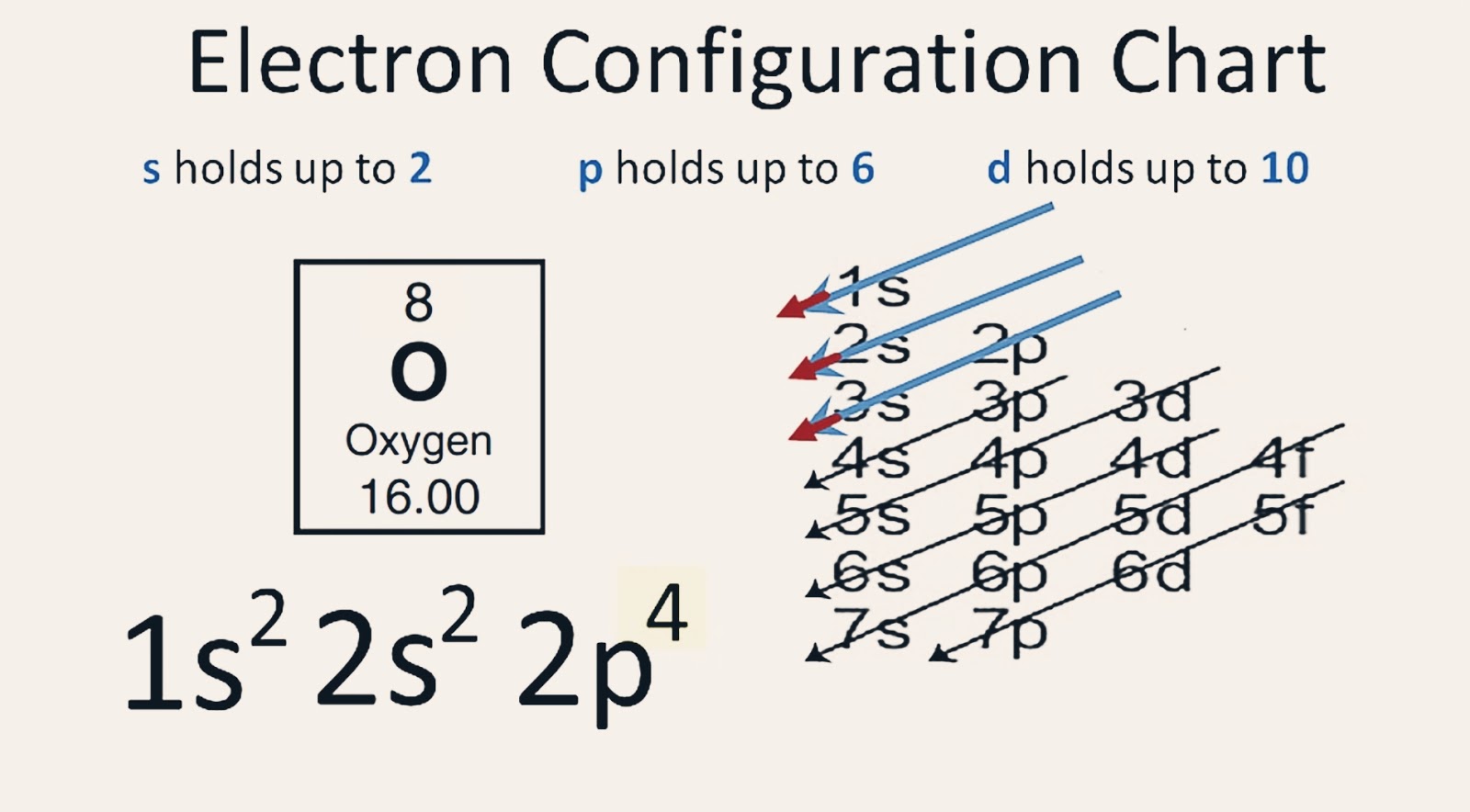
【5 Steps】Oxygen Electron Configuration in Just 5 Steps Electron Configuration of Oxygen(O)
The O atom has 2s 2 2p 4 as the electron configuration. Therefore, O has 2 unpaired electrons. Answer (b): The Br atom has 4s 2 3d 10 4p 5 as the electron configuration. Therefore, Br has 1 unpaired electron. Answer (c): The B atom has 2s 2 2p 1 as the electron configuration. Because it has one unpaired electron, it is paramagnetic.

Electron configuration of oxygen ion Lousiana
Electron Configurations. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with the loss of or gain.
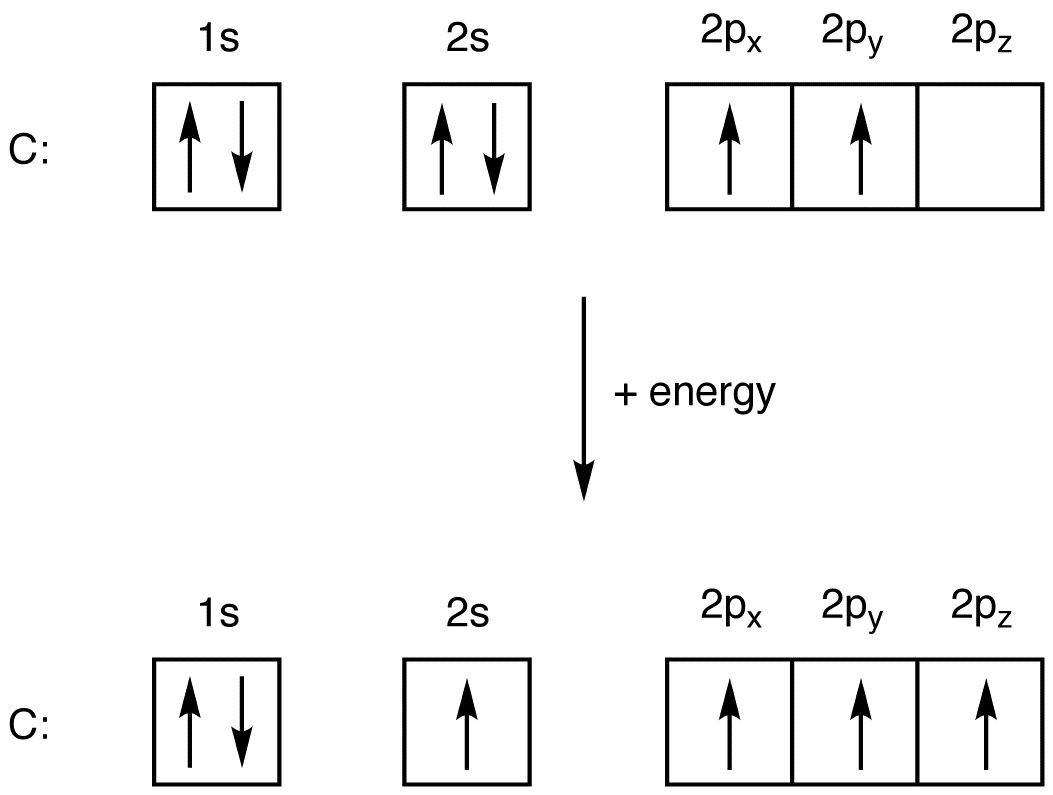
Oxygen Electron Configuration (O) with Orbital Diagram
Electron configurations are the summary of where the electrons are around a nucleus. As we learned earlier, each neutral atom has a number of electrons equal to its number of protons.
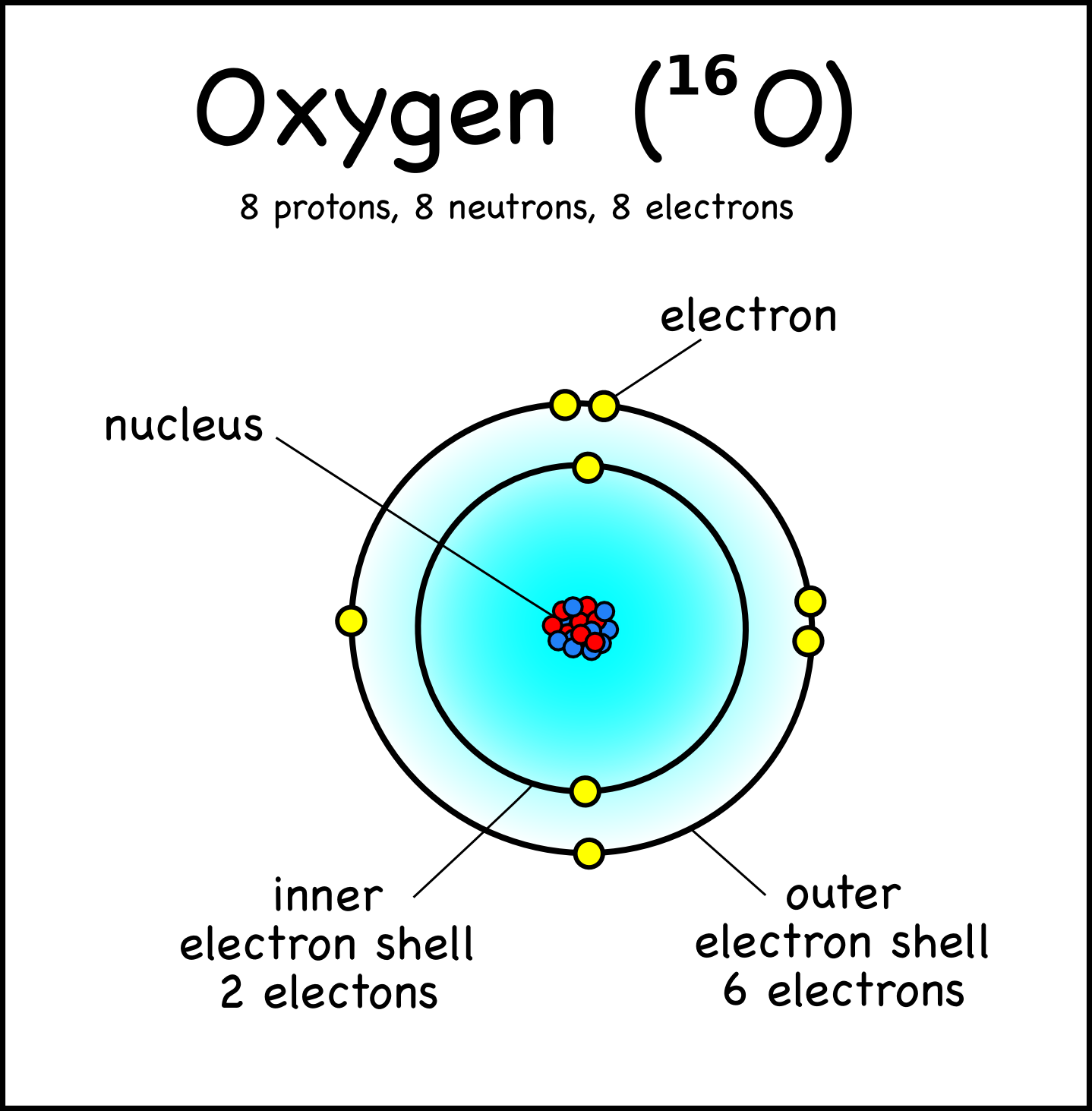
Drawing Atoms Montessori Muddle
The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number between 0 and 14. Thus in the building-up process for the lanthanoids.

Symbol and electron diagram for Oxygen Royalty Free Vector
The electronic configuration of an atom in the shell atomic model may be expressed by indicating the number of electrons in each shell beginning with the first. For example, sodium (atomic number 11) has its 11 electrons distributed in the first three shells as follows: the K and L shells are completely filled, with 2 and 8 electrons.
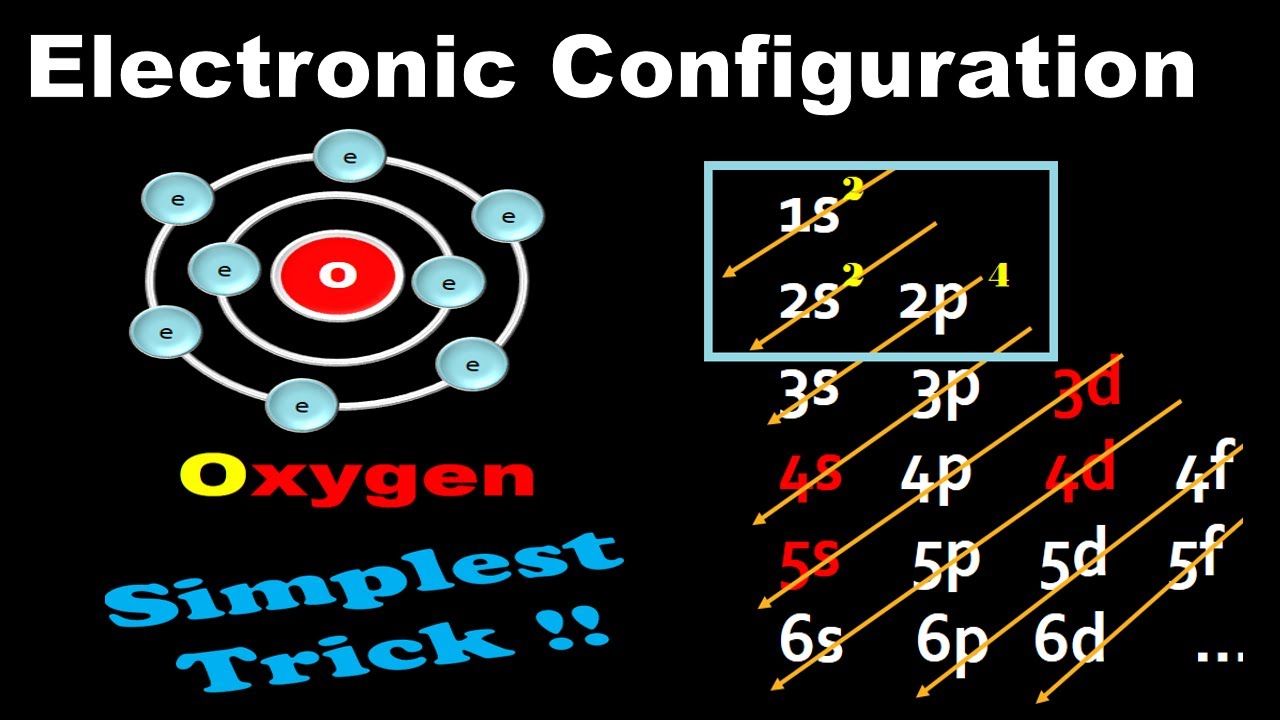
Electronic Configuration For Oxygen spdf Trick Chemistry Atomic Number 8 YouTube
The arrangement of electrons in oxygen in specific rules in different orbits and orbitals is called the electron configuration of oxygen. The electron configuration of oxygen is [ He] 2s 2 2p 4, if the electron arrangement is through orbitals. Electron configuration can be done in two ways. Electron configuration through orbit (Bohr principle)
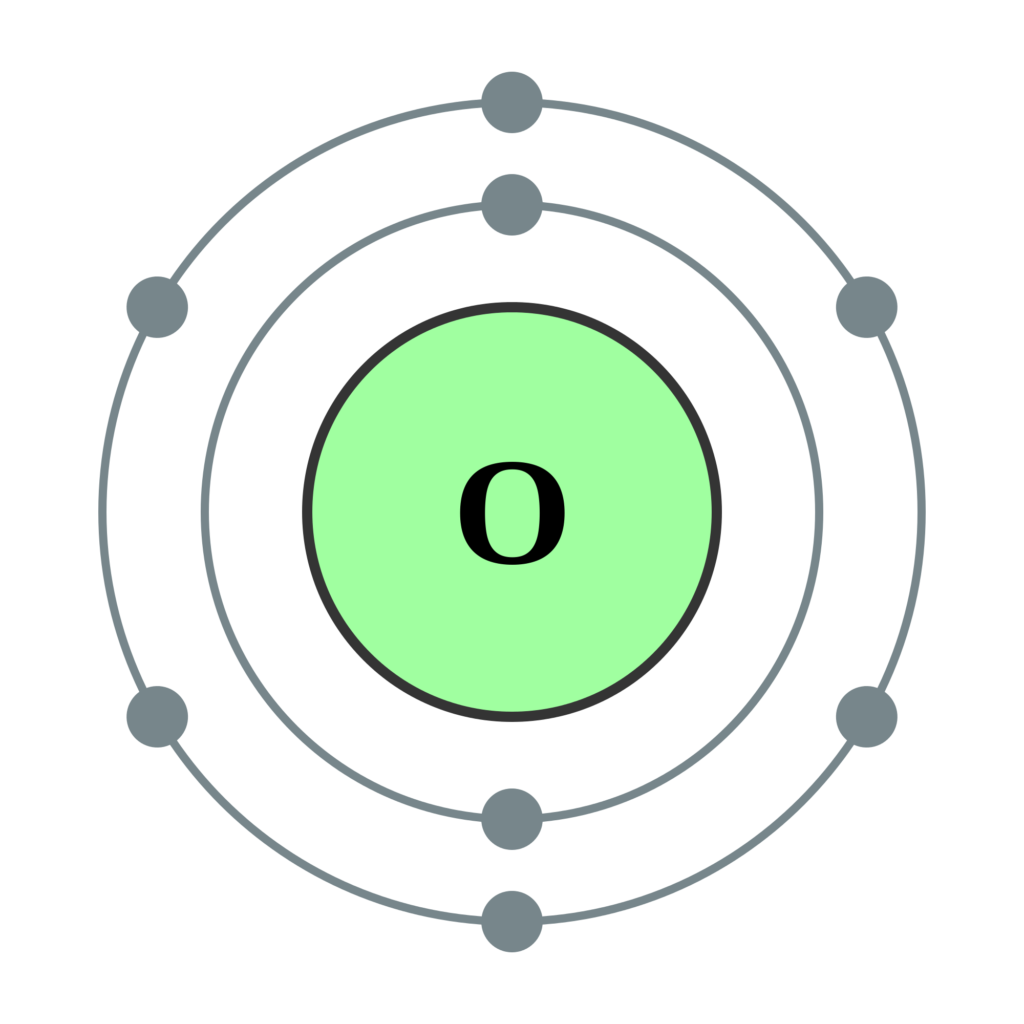
What is the Electron Configuration of Oxygen Archives Dynamic Periodic Table of Elements and
Electron configurations are a simple way of writing down the locations of all of the electrons in an atom. As we know, the positively-charged protons in the nucleus of an atom tend to attract negatively-charged electrons.

Oxygen Bohr Model (Diagram, Steps To Draw) Techiescientist
In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3.
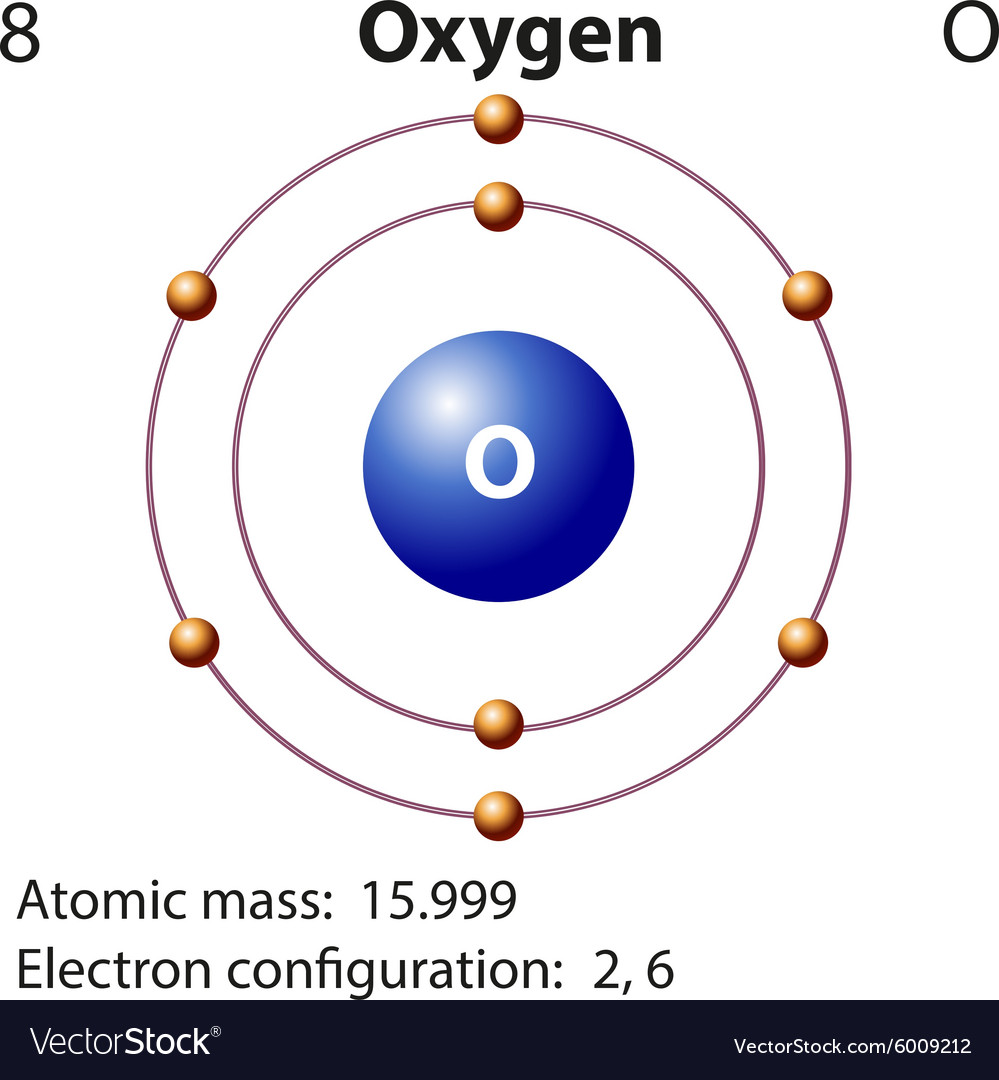
Diagram representation element oxygen Royalty Free Vector
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2). You will get the detailed information about the periodic table which will convert a newbie into pro. 3). You will also get the HD images of the Periodic table (for FREE).
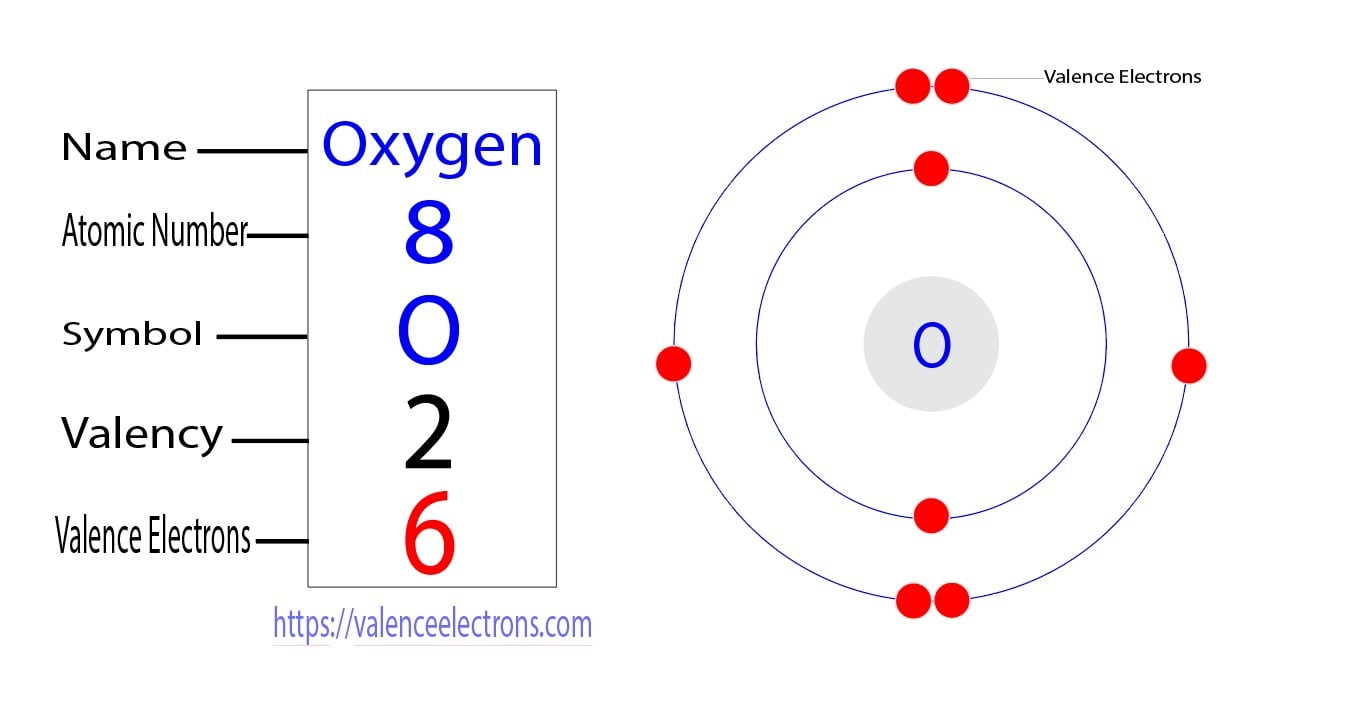
Electron Configuration for Oxygen (O, and O2 ion)
Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. - helps chemist understanding how elements form chemical bonds. - can be written using the period table or an electron configuration chart. How to Write the Electron Configuration for Oxygen Oxygen is the eighth element with a total of 8 electrons.
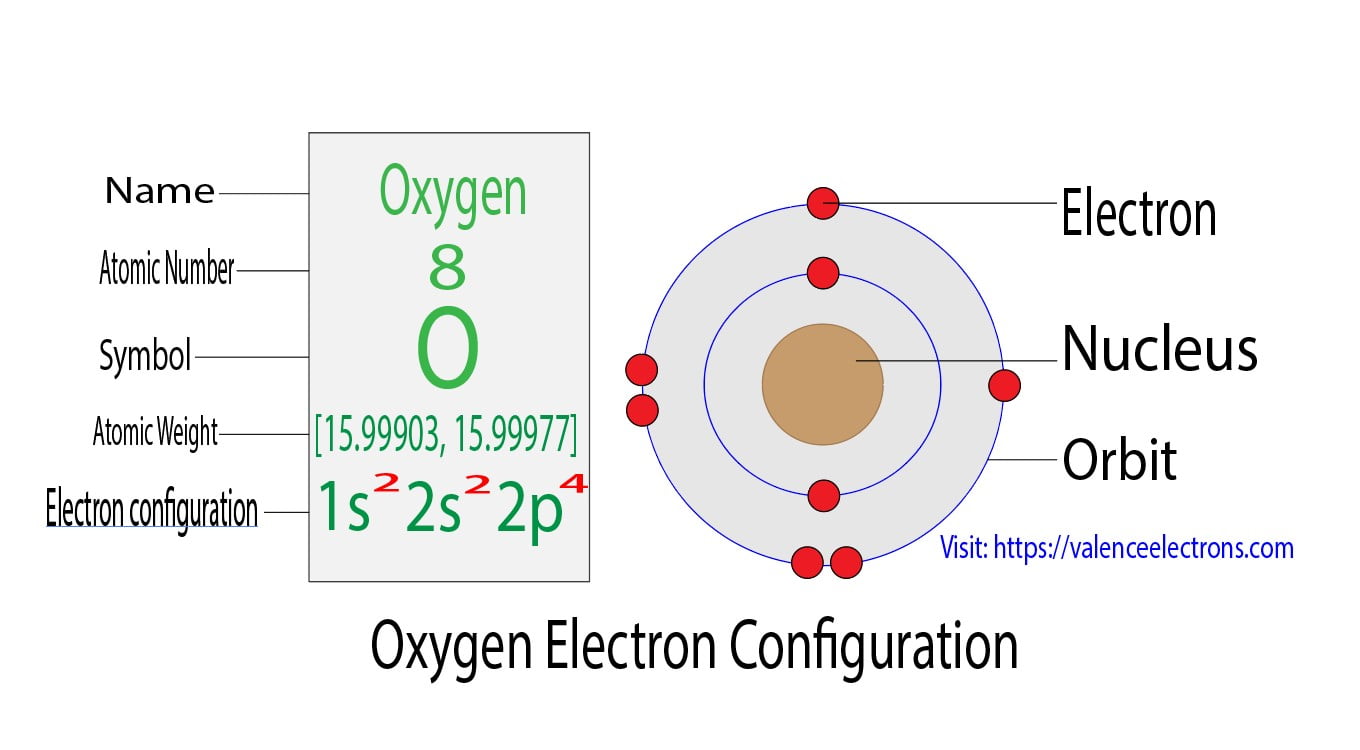
Electron Configuration for Oxygen (O, and O2 ion)
799 130K views 4 years ago In this video we will write the electron configuration for O 2-, the Oxide ion. We'll also look at why Oxygen forms a 2- ion and how the electron configuration for.

Electron Configuration Of Oxygen In Ground State
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure 6.25 ): The number of the principal quantum shell, n,

List of Electron Configurations of Elements
Oxygen Electron Configuration Wayne Breslyn 724K subscribers Join Subscribe Subscribed 683 Share 130K views 10 years ago A step-by-step description of how to write the electron configuration.
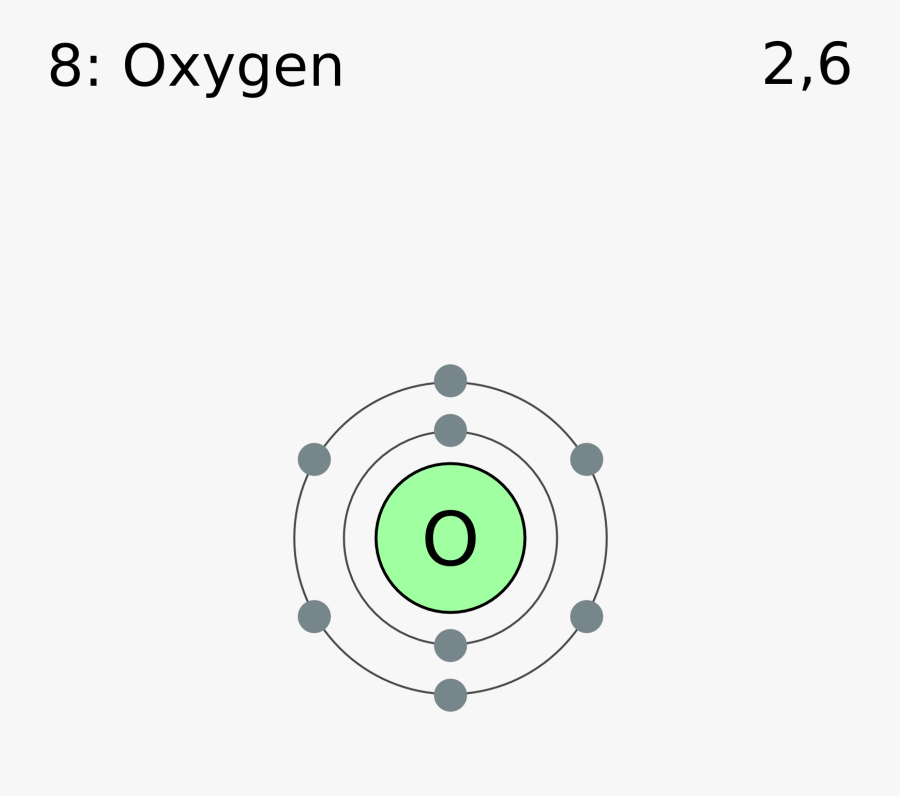
Electron Shell Diagram For Oxygen , Free Transparent Clipart ClipartKey
The electron configuration of an element describes how electrons are distributed in its atomic orbitals. Electron configurations of atoms follow a standard notation in which all electron-containing atomic subshells (with the number of electrons they hold written in superscript) are placed in a sequence.